Understanding electrochemical corrosion at metal-liquid nanointerfaces
The aim of the current study is to investigate electrochemical corrosion mechanisms by examining the metal-liquid nanointerfaces. To achieve this, corrosive fluids will be strategically trapped within metal structures using novel additive micro fabrication techniques. Subsequently, the nanointerfaces will be analyzed using cryo-atom probe tomography.
Corrosion is the gradual degradation of materials, which can also be driven by electrochemical reactions, where metal atoms lose electrons and form oxides and other compounds. Corrosion can lead to significant structural and functional failures in industrial applications, making its study crucial for materials science. Therefore, understanding corrosion in a fundamental and atomistic level will help better prepare, engineer and design sustainable materials in future.
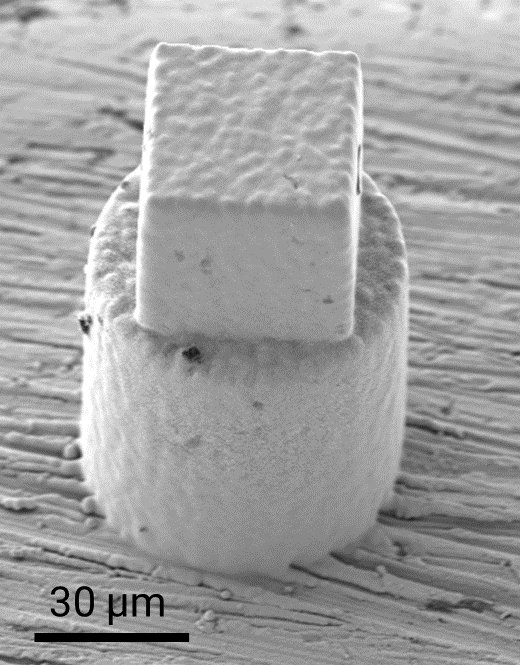
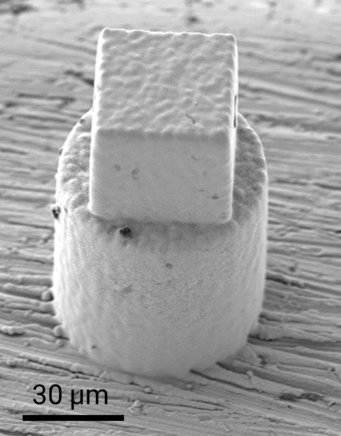
In this study, an advanced microfabrication technique based on localized electrodeposition in liquid (LEL) is utilized to print microscale copper cylinders with acidic liquid encapsulations. Following fabrication, the printed structures are cryo-frozen and subsequently, thin needles are extracted from these structures to analyze the chemical components present at the interface with atomic scale precision using atom probe tomography (APT).
Additionally, the structures are subjected to an external potential to accelerate the chemical reactions of the encapsulated liquid with the copper casing. Subsequently, a quick plunge freezing is done to freeze the chemical processes and nanosharp needles are again extracted, for a detailed APT observation of the modified metal-liquid interface. This approach provides valuable insights into the various electrochemical corrosion reactions occurring at the interface, contributing to the fundamental understanding required for the improved design of materials and structures. By studying the interactions with different chemically corrosive liquids, this research will elucidate the fundamental atomistic reactions, aiding in the development of enhanced mitigation strategies and more resilient materials in the future.