Impurity engineering for electrochemical nano-catalysts
Enabling a ‘hydrogen economy’ requires developing fuel cells satisfying economic constraints, reasonable operating costs and long-term stability. The fuel cell is an electrochemical device that converts chemical energy into electricity by recombining water from H2 and O2, allowing to generate environmentally-friendly power for e.g. cars or houses. However, upscaling anion-exchange membrane fuel cells (AEMFCs) is hindered by the slow kinetics of hydrogen oxidation reaction (HOR) at the anode.
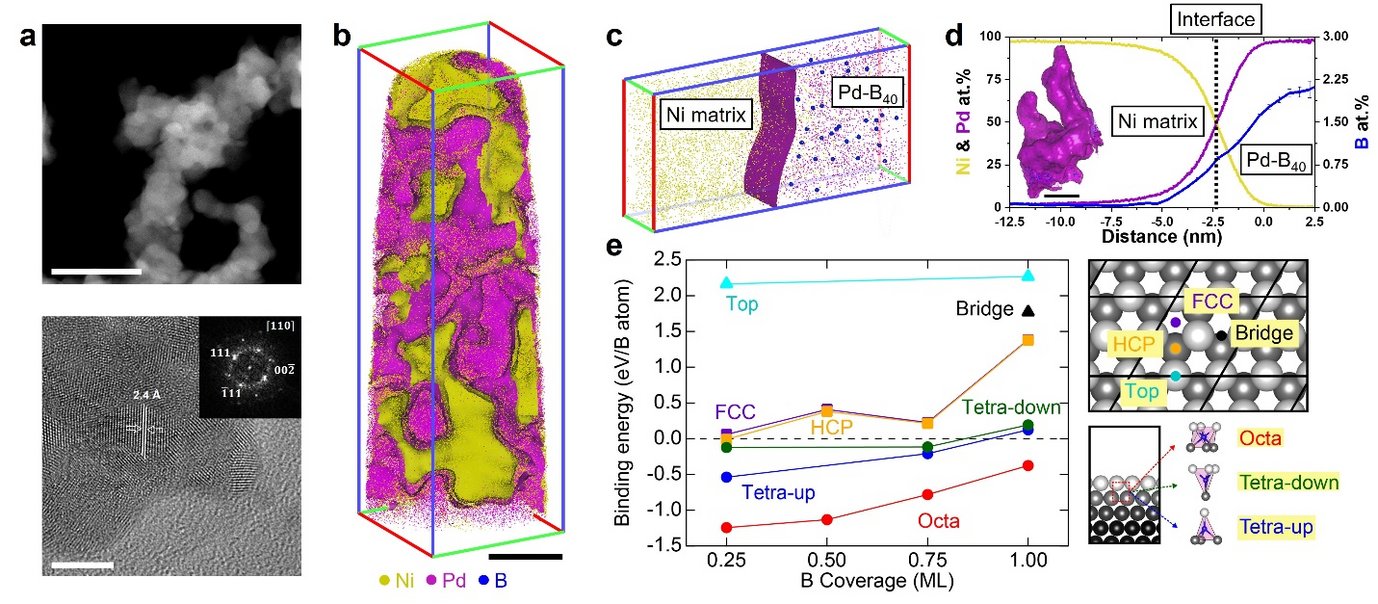
Here, we propose the impurity engineering approach to resolve the issue. First, we observe unwanted boron integration into a palladium nano-aerogels synthesised by wet-chemistry. We turn this into a materials design strategy, and use impurity engineering to synthesize electro-catalysts with a controlled boron-doping level, explained by ab-initio calculations. We show that the activity of the hydrogen oxidation in alkaline conditions increases by nearly two orders of magnitude with increasing boron-doping. We rationalise our findings using ab-initio calculations of hydrogen adsorption on boron-doped palladium. These insights from our combined experimental and theoretical investigation enable us to propose impurity engineering to design more efficient and stable catalysts in the future.