Interplay of chemistry and faceting at grain boundaries in a high strength Al alloy
Grain boundaries (GBs) are regions connecting adjacent crystals with different crystallographic orientations. GBs are a type of lattice imperfection, with their own structure and composition, and as such impact a material’s mechanical and functional properties. Structural motifs and phases formed at chemically decorated GBs can be of a transient nature or are local thermodynamic structural-chemical equilibrium states.
The boundary between two crystal grains can decompose into arrays of facets with distinct crystallographic characters. Faceting occurs to minimize the system’s free energy, i.e., when the total interfacial energy of all facets is below that of the topologically shortest interface plane. Previously, the interplay of chemistry and faceting was revealed for specific GBs in well-defined bicrystals, which are realistically not encountered in engineering alloys. The majority of GBs in bulk metallic materials from conventional processing is of a more general type. Evidence for structural transitions in such boundaries in Al-alloys is scarce, and the interplay with chemistry is not well understood. Recently [1], we have revealed the subtle yet important interplay between the faceting of GBs and their chemical decoration with solutes in a high strength 7xxx Al alloy. We combined cutting-edge microscopy, microanalysis techniques, and atomistic simulations to demonstrate that facets exist in polycrystalline samples, ranging in length from a few to hundreds of nanometres at GBs. We also evidenced that the local chemistry is strongly correlated with the facet character.
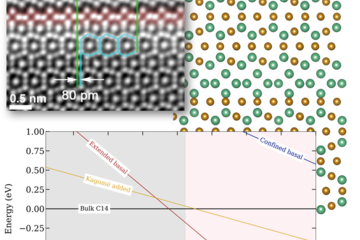
In the present report, we highlight one example of the near Σ5 coincident site lattice boundaries in a coarse grained 7xxx Al alloy. A homogenized Al-6.22 %Zn-2.46 %Mg-2.13 %Cu (wt. %) was used for this study. The alloy was solution heat treated at 475 °C, followed by water quenching. The detailed process and precipitation behaviour during aging is described in [2]. The Σ5 grain boundary observed has a misorientation angle of 38o about a <001> common axis. Fig. 1(a) illustrates the Σ5 GB characterized by Z-contrast high-angle annular dark-field (HAADF) scanning transmission electron microscopy (STEM) performed along the common tilt <001> Al zone axis. The GB configuration contains two sets of alternating facets along the entire length, with each type highlighted by a coloured line. The orange segments are symmetric {120} facets, and the green segments are asymmetric {130} planes. Fig. 1(b) shows the energy dispersive spectroscopy (EDS) analysis from the faceted Σ5 GB. At this scale, we do not observe segregation at the {130} facets, while the {120} facets bear roughly 300 nm lathlike precipitates enriched with Mg and Cu. The HAADF-STEM image in Fig. 1(c) reveals that the precipitate has an orthorhombic structure, which matches the S phase (Al2CuMg). The S phase is semi-coherent with the Al-matrix and has an orientation relationship of <100>s//<100>Al, and an interface of {001}S//{120}Al.
![Fig. 1: (a) HAADF-STEM image of a near Σ5 GB in the as-quenched Al-Zn-Mg-Cu alloy; (b) EDS maps of the faceted GB showing Mg and Cu enriched precipitates; (c) HAADF-STEM image of the precipitate highlighted by blue rectangle in (a), with inset of the corresponding Fast Fourier transform pattern. GB: grain boundary. Figure is from Ref. [1], published under a CC-Attribution 4.0 international license.](/4695339/original-1643028329.jpg?t=eyJ3aWR0aCI6MTM3OCwib2JqX2lkIjo0Njk1MzM5fQ%3D%3D--1761dcbb3772de54417ef4c67f96007afeec30ea)
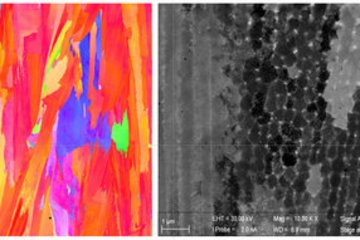
We performed complementary atom probe tomography (APT) analyses on the same Σ5 GB on another location, shown in Fig. 2. Two lathlike S phases enriched in Mg and Cu are visible in Fig. 2(a), highlighted by isosurfaces delineating regions containing more than 10 at. % Mg. A composition profile across the large precipitate was calculated along a 25 nm-diameter cylinder aligned with the red arrow in Fig. 2(b). It shows an average composition of 52 at. % Al, 23 at. % Mg, 18 at. % Cu, and 7 at. % Zn within the precipitate. This agrees with the stoichiometry of the S phase (Al2CuMg). It is most likely that the 7 at. % Zn substitutes to Cu sites, as Cu and Zn are both undersized, and the combined compositions of Cu (18 at. %) and Zn (7 at. %) equals 25 at. %, as expected for the Al2Mg(Cu/Zn) stoichiometry. At this more detailed scale, we also observe Zn, Mg, and Cu enrichments along the {130} facet that connects the two precipitates and the corresponding composition profile is plotted in Fig. 2(c). The average composition in the enriched region are 3.1 at. % Zn, 3.1 at. % Mg and, 0.5 at. % Cu, which are enrichment factors of two in Zn and Mg and 3 in Cu compared to the abutting bulk solute composition. Combining the STEM and APT results, we conclude that the S phase nucleates on the {120} facets, whereas solutes segregate to the {130} facets of the faceted Σ5 GB.
![Fig. 2: APT analysis of the same Σ5 GB with STEM analysis: (a) Atom maps of all elements; (b) Corresponding composition profile across the precipitate calculated along the red arrow; (c) Corresponding composition profile across the region between two precipitates calculated along the purple arrow. GB: grain boundary. Figure is from Ref. [1], published under a CC-Attribution 4.0 international license.](/4695370/original-1643028329.jpg?t=eyJ3aWR0aCI6MTM3OCwib2JqX2lkIjo0Njk1MzcwfQ%3D%3D--732c0e4604d399f03a3f4fe7333ae7f51f6cecd4)
We then used atomistic simulations to better understand the interplay of the chemistry and the structural faceting of the Σ5 GB. We examined the formation of a Mg-rich phase near the symmetric {120} and {130} 36.87° <001> Σ5 GBs by using the Variance Constrained Semi-Grand Canonical Molecular Dynamics/Monte Carlo (MD/MC) approach [3]. Both GBs experience various stages of segregation, from the initial decoration of the GB and adjacent planes, to the nucleation of a Mg-rich precipitate. However, the underlying energy landscape of the {120} boundary drives the precipitate to grow in perfect coherence with the decorated GB plane. In contrast, at the {130} boundary the precipitate retains double-Al-layer defects even after many MC swaps. These computational predictions rationalize that large Mg-rich phase form at each {120} facet, while the {130} facets experience only local segregation enrichment.
We also evidenced that the self-consistent co-evolution of facet structure and chemistry leads to the formation of periodic segregation patterns of 5 – 10 nm at Σ11, and Σ13a GBs [1]. Although the character of individual GB is often overlooked in the segregation study in engineering Al alloys, our study enriches the current understanding of the chemical-structural interactions. The solute segregation and heterogeneous precipitation at GBs have an impact on the corrosion properties of Al alloys. Within the same GB, the facets coupled with the anisotropic segregation and the precipitation behaviour will significantly influence the GB cohesion, intergranular fracture, and corrosion resistance of engineering Al alloys. Better predictions for the temporal evolution of the alloy’s microstructure during aging and in service will require knowledge of the solute distribution within the microstructure, particularly at GBs.
References
- Zhao, H.; Huber, L.; Lu, W.; Peter, N.; An, D.; De Geuser, F.; Dehm, G.; Ponge, D.; Neugebauer, J.; Gault, B.; Raabe, D.: Phys. Rev. Lett. 124 (2020) 106102.
- Zhao, H.; De Geuser, F.; Kwiatkowski da Silva, A.; Szczepaniak, A.; Gault, B.; Ponge, D.; Raabe, D.: Acta Mater. 156 (2018) 318.
- Sadigh, B.; Erhart, P.; Stukowski, A.; Caro, A.; Martinez, E.; Zepeda-Ruiz, L.: Phys. Rev. B 85 (2012) 184203.
![Fig. 1: (a) HAADF-STEM image of a near Σ5 GB in the as-quenched Al-Zn-Mg-Cu alloy; (b) EDS maps of the faceted GB showing Mg and Cu enriched precipitates; (c) HAADF-STEM image of the precipitate highlighted by blue rectangle in (a), with inset of the corresponding Fast Fourier transform pattern. GB: grain boundary. Figure is from Ref. [1], published under a CC-Attribution 4.0 international license. Fig. 1: (a) HAADF-STEM image of a near Σ5 GB in the as-quenched Al-Zn-Mg-Cu alloy; (b) EDS maps of the faceted GB showing Mg and Cu enriched precipitates; (c) HAADF-STEM image of the precipitate highlighted by blue rectangle in (a), with inset of the corresponding Fast Fourier transform pattern. GB: grain boundary. Figure is from Ref. [1], published under a CC-Attribution 4.0 international license.](/4695339/original-1643028329.jpg?t=eyJ3aWR0aCI6ODQ4LCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6NDY5NTMzOX0%3D--a26af6260fce6d3a9d07522b453e5eb2bbd871d5)
![Fig. 2: APT analysis of the same Σ5 GB with STEM analysis: (a) Atom maps of all elements; (b) Corresponding composition profile across the precipitate calculated along the red arrow; (c) Corresponding composition profile across the region between two precipitates calculated along the purple arrow. GB: grain boundary. Figure is from Ref. [1], published under a CC-Attribution 4.0 international license. Fig. 2: APT analysis of the same Σ5 GB with STEM analysis: (a) Atom maps of all elements; (b) Corresponding composition profile across the precipitate calculated along the red arrow; (c) Corresponding composition profile across the region between two precipitates calculated along the purple arrow. GB: grain boundary. Figure is from Ref. [1], published under a CC-Attribution 4.0 international license.](/4695370/original-1643028329.jpg?t=eyJ3aWR0aCI6ODQ4LCJmaWxlX2V4dGVuc2lvbiI6ImpwZyIsIm9ial9pZCI6NDY5NTM3MH0%3D--d08951c3fe414a68bf2611f5b6698bdb5913e188)