Oxide formation
The example of oxide on metallic zinc: electronic structure and formation
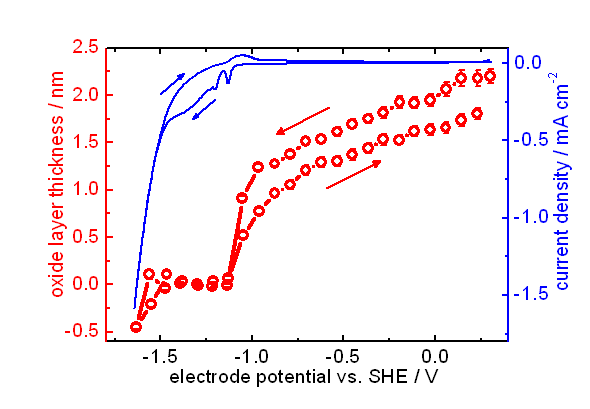
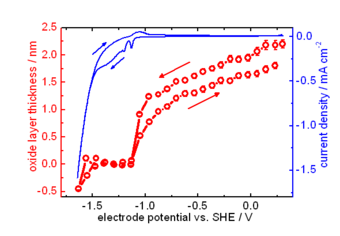
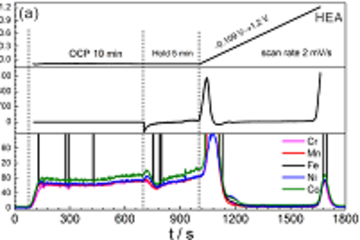
Zn is one of the most important metals in applications. Due to its oxidation propensity, Zn is a very efficient sacrificial anode in cathodic protection, and used as metallic coating. Corrosion products of Zn metal and its coatings include zinc oxide, zinc hydroxide and carbonates. The electronic properties of the mostly semiconducting oxide layer play an important role in the corrosion behaviour of Zn. We use experimental investigation of properties, such as thickness and band gap, of the forming oxide films. The particular challenge in case of thin films forming in the initial stages of corrosion processes is that they are highly disordered, transient species. Formation and evolution of such structures can be conveniently analysed using optical reflection spectroscopy, as the optical absorption spectrum is directly related to the electronic structure of the film. Often, besides electrochemical techniques, we use spectroscopic ellipsometry to study the initial stages, as well as the evolution with time, of layer thickness and layer absorption spectrum, in controlled atmospheres and in electrolyte.
Publications:
•Ying Chen, Paul Schneider, Bi-Ju Liu, Sergiy Borodin, Bin Ren, Andreas Erbe: Electronic structure and morphology of dark oxide on zinc generated by electrochemical treatment. Physical Chemistry Chemical Physics, 15, 9812-9822 (2013).
•Ying Chen, Andreas Erbe: In-situ spectroscopic ellipsometry during electrochemical treatment of zinc in alkaline carbonate electrolyte. Surface Science, 607, 39-46 (2013).
•Ying Chen, Paul Schneider, Andreas Erbe: Investigation of native oxide growth on zinc in different atmospheres by spectroscopic ellipsometry. Physica Status Solidi A, 209, 846-853 (2012)
•Juan Zuo, Andreas Erbe: Optical and electronic properties of native zinc oxide films on polycrystalline Zn. Physical Chemistry Chemical Physics, 12, 11467-11476 (2010).
The example of oxide on copper
With the possibility to form Cu2O and CuO, copper offers a richer surface chemistry than zinc. In situ analysis and a comparison with simulations shows that during free corrosion in biological buffers, predominantly CuO is forming.
Publications:
•Michael Hans, Andreas Erbe, Salima Mathews, Ying Chen, Marc Solioz, Frank Mücklich: Role of copper oxides in contact killing of bacteria. Langmuir, 29, 16160-16166 (2013).