Electrified water/solid interfaces
Our project aims at obtaining a detailed atomistic understanding of electrochemical interfaces from first principles.
The chemistry of water/solid interfaces is essential to understand the precise atomistic mechanisms in corrosion, heterogeneous catalysis, electrochemistry, geology and atmospheric chemistry. Solar water splitting and Power-to-X concepts are highly promising approaches for converting sunlight into chemical fuels, but suffer from severe stability and corrosion problems. Understanding and mitigating these problems requires precise information at the atomistic level on the structure of electrode/electrolyte interfaces, also under bias, the solvation of surfaces and ions and on the electrochemical processes occurring at these interfaces.
We investigate interfaces between liquid water and elemental semiconductors or metal(-oxide) surfaces as model systems to understand fundamental processes and principles at electrochemical interfaces. Using a surface science type of approach, we focus on well-defined model systems to develop and validate methods that allow us to bridge the gap between ab initio theory and experiments.
Based on the "modern theory of polarization" [M. Stengel et al., Nature Physics 5, 304 (2009)], we perform ab initio MD simulations with applied electric fields. The electronic polarization is described within periodic boundary conditions using a Wannier function and efficient simultaneous diagonalization approach. At present, our calculations are performed at constant dielectric displacement. In the future, this aproach will be linked to our recently proposed thermopotentiostat.
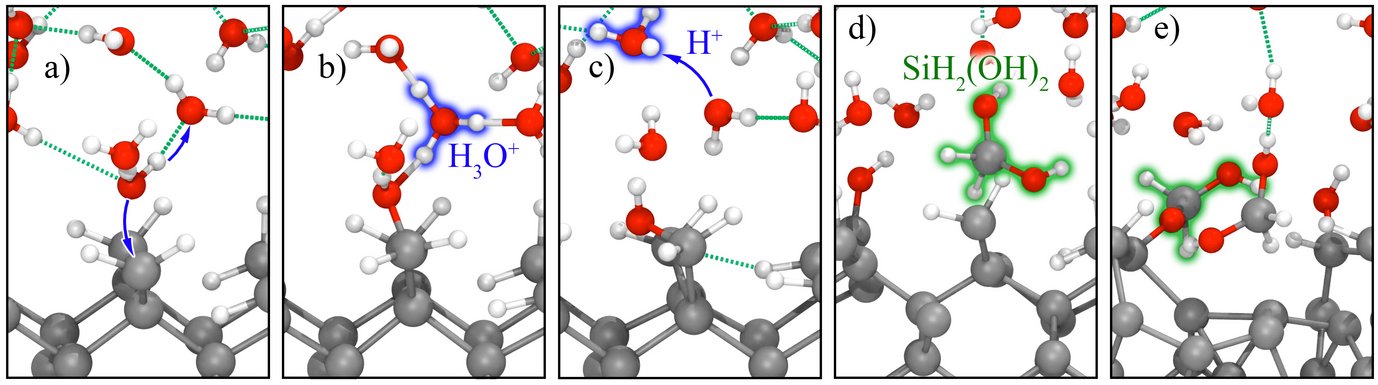
Using our finite field technique, we performed ab initio MD simulations for electrified interfaces between single-crystalline elemental semiconductors and liquid water. Without applied fields, these interfaces are stable at the time scales accessible to our simulations. Under anodic polarization (positive charge on the surface), the hydrogenated Si(100)/water interfaces quickly starts to oxidize.
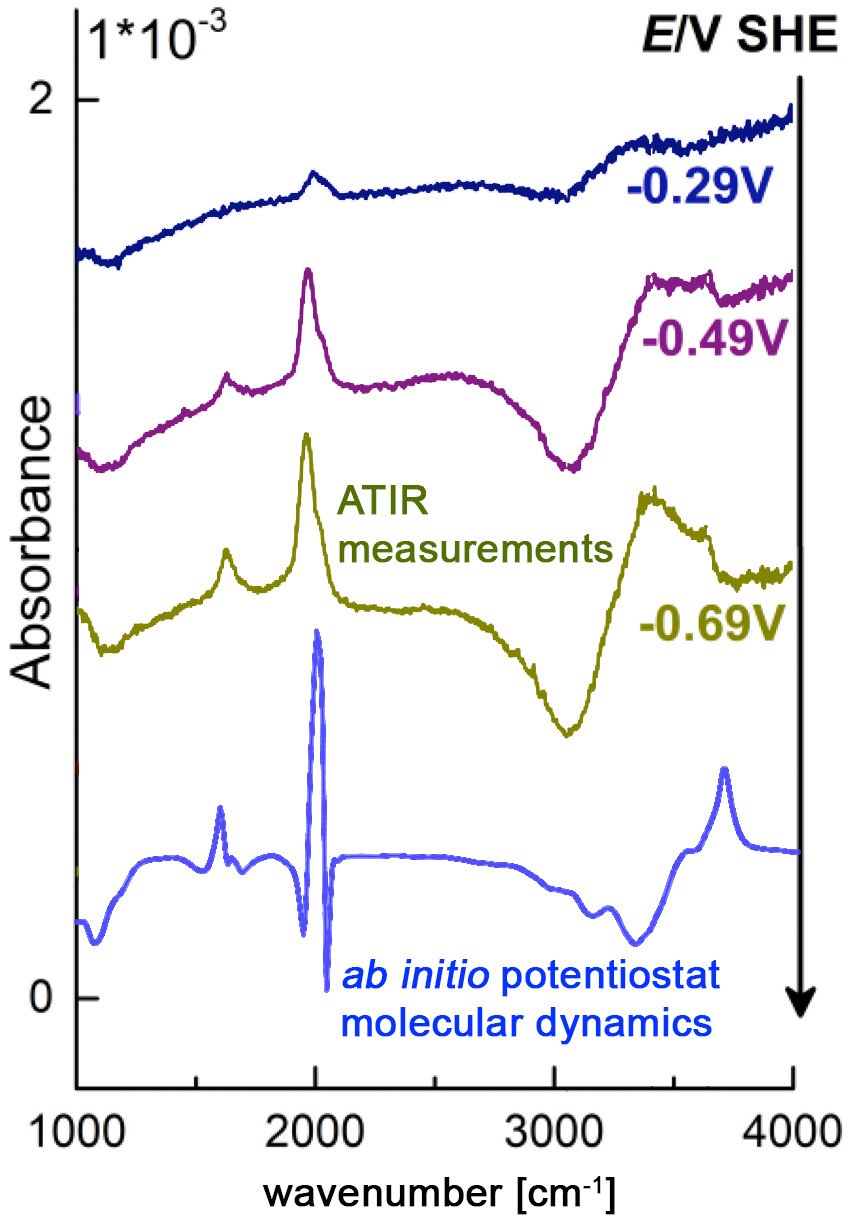
MD simulations of this type allow us to identify detailed reaction mechanisms and intermediates. The applied electric bias is then adjusted to stabilize intermediates of interest, so that vibrational spectra can be calculated from MD simulations and the maximum entropy method. By comparing to optical fingerprints from attenuated total internal reflection (ATR-IR) spectroscopy, we validate our methods and interpret experiments. This work is performed in close collaboration with the Interface Spectroscopy Group.














