Influence of Microstructure on Hydrogen Embrittlement in Cr/Mn-Containing High-Mn Steels
It is well-documented that already small amounts of hydrogen have a detrimental effect on the mechanical properties of many metals and alloys, especially in terms of a significant ductility loss. In metallic systems, such as high-Mn steels, the delayed fracture caused by hydrogen is particularly difficult to control. Hydrogen that is incorporated in the material during manufacturing, processing, or during service time can get trapped at various lattice defects like dislocations, microvoids, grain boundaries, and interfaces and can slowly be released to critically stressed regions in the microstructure [1].
In steel design, there has recently been much interest in the role of carbides. They contribute to the strengthening of the materials. On the other hand, they can serve as microstructural traps for hydrogen. The presence of well designed hydrogen trapping sites could be a relevant strategy to make the steels less susceptible to HE. At the same time, the accumulation of hydrogen at the carbide-matrix interface can cause a crtical local hydrogen concentration giving rise to crack initiation. Ab-initio simulation techniques are ideally suited to adjust the amount of H trapping by modifying the chemical composition of the steels at an atomistic scale [2].
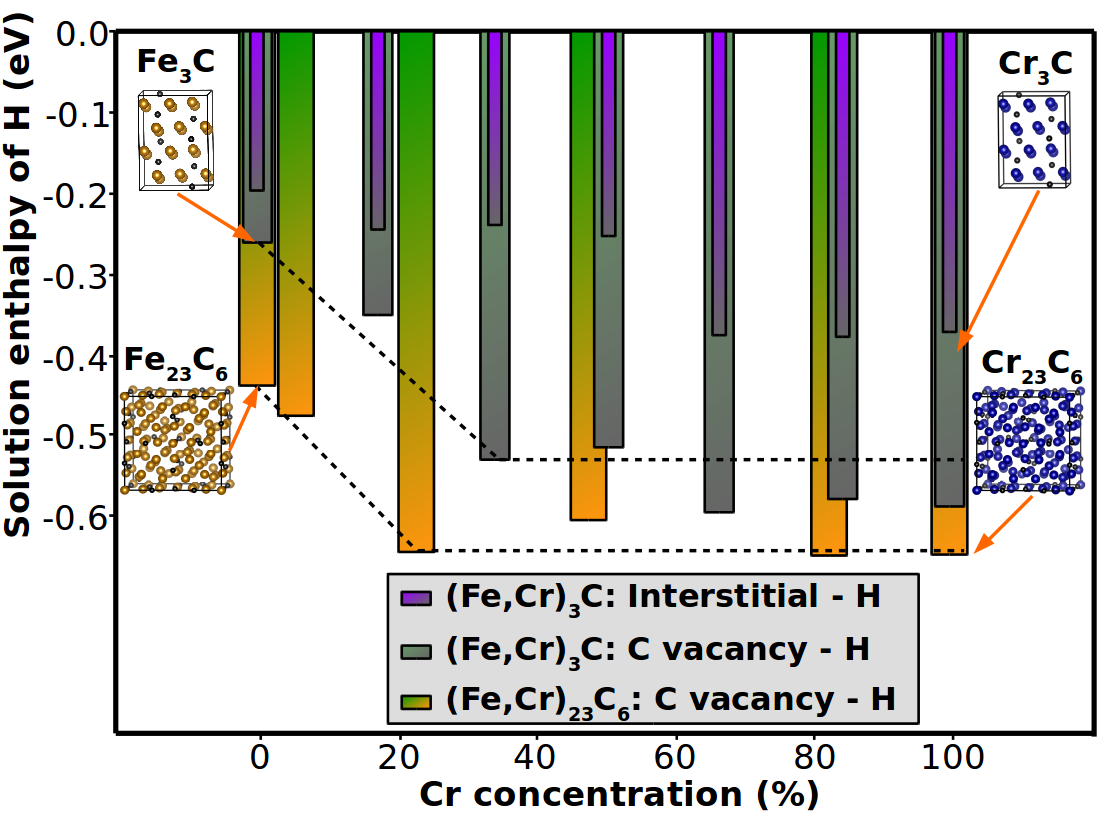
Ab initio calculated solution enthalpy of hydrogen at interstitial and C vacancy positions in Cr-containing (Fe,Cr)23C6 and cementite with increasing concentration of Cr. The H incorporation into interstitial sites and at C vacancies are compared.
The project deals with high-Mn steels that contain pearlitic microstructures due to the presencce Cr/Mn containing iron carbides like (Fe,Cr,Mn)23C6. A major aspect is to explore the impact of these alloying elements on the H solubility in Cr containing carbides. The insights are subsequently extrapolated to the complex interface between the carbides and the matrix, in order to evaluate possible decohesion mechanisms [3]. As an example, the figure demonstrates the chemical trends for H in cementite and (Fe,Cr)23C6. If incorporated into a C vacancy, the solubility of H is found to increase with Cr content in the nearest-neighbor shell, while the trends are less systematic for interstitial positions. A similar behaviour is found for the ferrite-cementite interface with and without Cr. Based on these insights, it is a goal of the project to develop strategies for making high-Mn steels less susceptible to HE.












