Damage Analysis in Hydrogen embrittlement
Understanding hydrogen-assisted embrittlement of advanced structural materials is essential for enabling future hydrogen-based energy industries. A crucially important phenomenon in this context is the delayed fracture in high-strength structural materials.
Factors affecting the hydrogen embrittlement are the hydrogen content, residual stress (as well as applied stress) and microstructure. These factors can all lead to a critical condition for hydrogen embrittlement through specific mechanisms, e.g. hydrogen-induced decohesion, hydrogen-enhanced strain-induced vacancy formation and hydrogen-enhanced localized plasticity. In our group at the Max-Planck Institut in Düsseldorf we study the mechanisms of hydrogen embrittlement in a new class of steels, namely Fe–Mn–C twinning-induced plasticity (TWIP) steels. These materials present a new group of advanced high-strength and formable austenitic steels with high potential for automotive forming applications. Moreover, energy-related infrastructures and pipelines are envisaged as future applications of these steels. The hydrogen - promoted embrittlement of steels depends on the kind of steel and also on the hydrogen charging conditions. Phases such as soft ferrite, hard C-martensite, maraging-martensite, austenite etc. might follow different mechanism combinations, kinetics, and trapping spectra. Several important mechanisms were proposed: Hydride-induced embrittlement, i.e. effect of a second phase; Hydrogen-enhanced decohesion, HEDE, leading to brittle fracture; Hydrogen-enhanced localized plasticity, HELP, leading to ductile fracture; Hydrogen-enhanced vacancy formation and stabilization through the defectant mechanism.
Our current understanding of failure in many pipeline and sheet steels relates to the interaction among these different phenomena, characterized by these steps:
- i) H enhances dislocation formation leading to high dislocation densities and dense configurations;
- ii) the dislocation interactions can create vacancies (e.g. jog drag and the associated climb osmotic effects);
- iii) these vacancies get decorated and stabilized by hydrogen (it reduces their free energy of formation due to the defectant theory);
- iv) these nanovoids can coalesce and condensate at interfaces, interface junctions, microbands, twins, or cell walls;
- v) the voids can grow further to form larger voids.
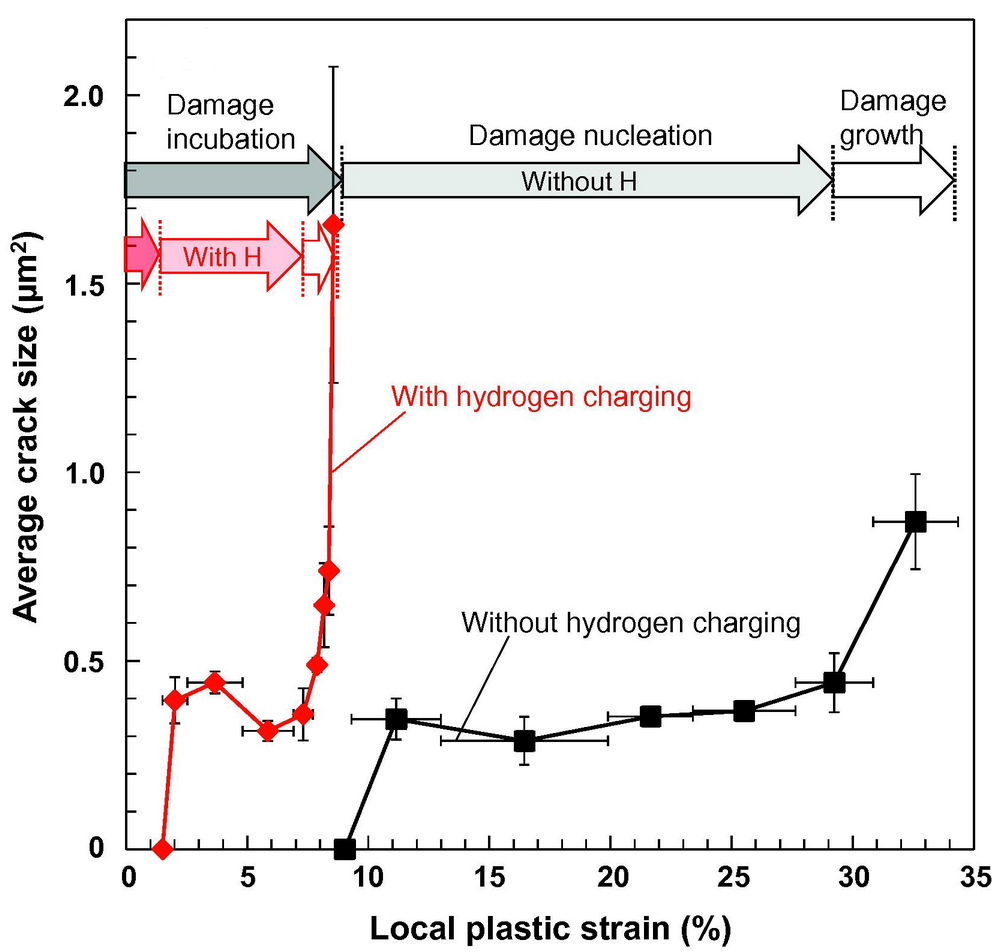
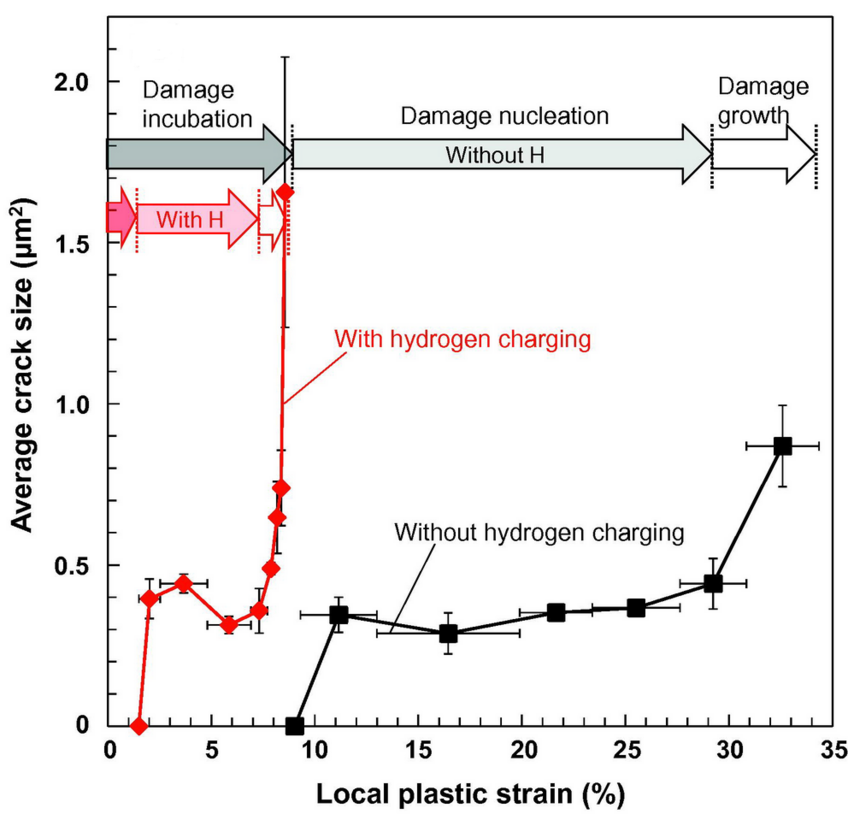
Damage evolution curves plotted against local plastic strain with and without hydrogen charging: average crack size