Interfacial processes/reactions at the atomic scale
The research group focuses on interfacial reactions and processes at liquid/solid interfaces on the atomic scale, in structural, energy and functional materials.
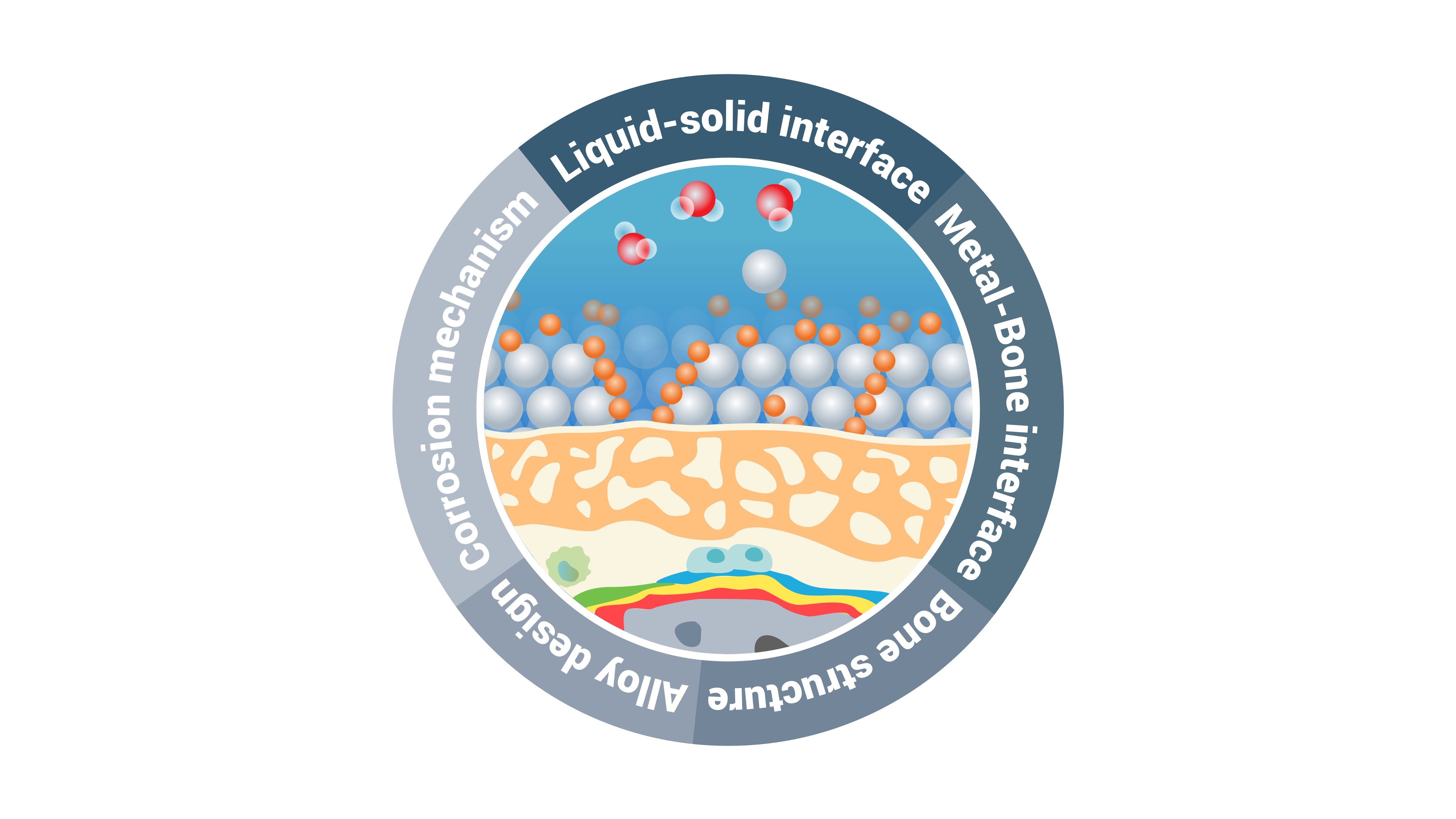
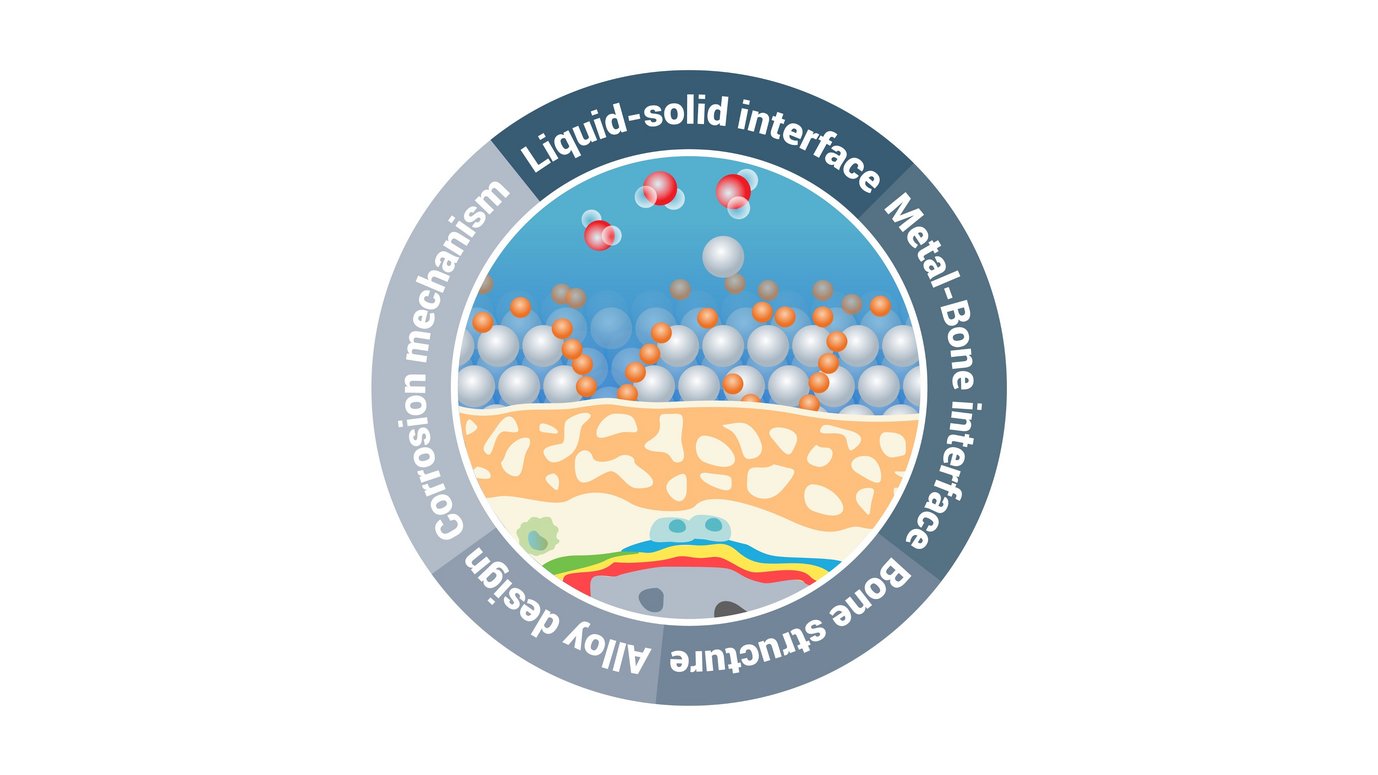
Why are liquid-solid interfaces generally of interest? Because the properties of materials are controlled through dynamic processes and reactions at these interfaces. One of the most critical aspects is the degradation of materials at liquid-solid interfaces, which leads to the failure of the materials' function and performance, e.g. in batteries, catalyst or of structural and functional materials. Understanding the chemical composition and structure of interfaces at the smallest length scale has an enormous potential from economic, societal and sustainability perspectives. However, analytical difficulties hinder the characterization of liquid-solid interfaces at the atomic level with a high chemical sensitivity and limits our understanding of interfacial reactions and processes at the liquid-solid interface. We have developed a new (quasi-)in-situ method using cryo-atom probe tomography to analyze the liquid-solid interface. We focus on observations at the atomic level, which allow us to study the structural and chemical interplay of material degradation at these interfaces. Such dynamic observations down to the atomic scale, combined with theoretical approaches, allow us to elucidate the underlying mechanisms and to optimize materials based on these findings.
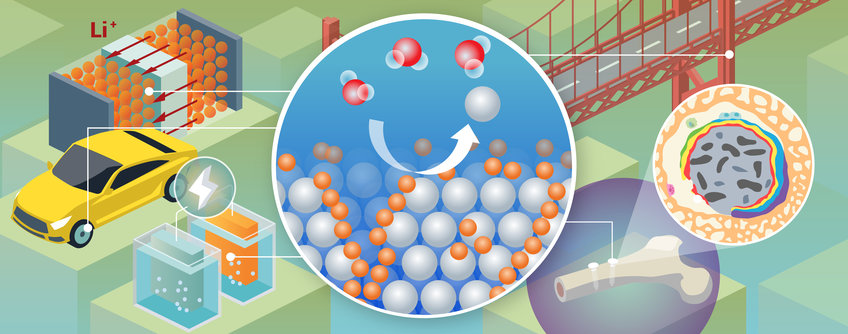
The importance of liquid-solid interfaces has increased significantly in recent years, linked to current endeavors towards the electrification of combustion processes (batteries) or to production of hydrogen (catalyst) aiming for a zero net-carbon emission by 2050. However, corrosion of metals is often neglected when considering reducing CO2 emissions. The replacement of corroded steel alone costs US$6 trillion per year and contributes approximately up to 9.1% of global CO2 emissions. The interactions and chemical exchanges at liquid-solid interfaces, particularly in the early stages of corrosion, remain poorly understood and new insights and knowledge is required to more effectively prevent and protect against corrosion.
Our focus is on the analyzing of the dynamic interplay between structure and composition at the atomic level. These efforts aim to better understand interfacial processes and reactions, and to control/prevent degradation of materials and their properties, thus contributing to the development of more advanced materials for a more sustainable future. Our research interests can be divided into three main areas: degradation of structural materials, functional materials and biological interfaces.
Mg alloys are a key topic of interest. Mg has a high strength-to-weight ratio ideal for lightweight structural applications, it is also biocompatible and biodegradable, making it an excellent candidate for temporary implants. Both applications are limited by rapid and uncontrollable corrosion. There are remaining knowledge gaps regarding the influence of alloying elements and their segregation at interfaces, and the influence of different electrolytes on the corrosion mechanism. Our newly developed cryo-atom probe tomography approach can bring unique insights by analyzing the liquid-solid interface quasi-(in-situ). The same approach can be used to study corrosion of other alloy systems, particularly aluminum alloys and steels, to advance the understanding of corrosion mechanisms and improve their performance and durability.
Additionally, our group is investigating the interplay between these corrosion phenomena and biological interfaces to understand their impact on the degradation of metallic implants and the biomineralization process of new bone formation. Biodegradable implants are a complex field involving a combination of metallurgy (alloy design, microstructure, microstructure, corrosion behavior) and biology (biocompatibility with cells and surrounding tissues, formation of new bone structures). The bone-implant interface is largely responsible for controlling the biodegradability of the metallic implant and enables the adaptation of the implant to the host tissue. Our group focuses on the analysis of these interfaces, especially for temporary implants, to better understand which processes at the interface at the atomic level control the corrosion behavior and how the release of ions drives the biomineralization process to fill the knowledge gap. These new insights will improve our understanding of reactions at metal-biological interfaces.
We will use our newly developed cutting-edge approaches to study the early-stage degradation mechanisms at liquid-solid interfaces at the near-atomic scale on a range of structural and functional materials. Cryo-atom probe tomography will be complemented by cryo- and conventional electron microscopy and spectroscopy methods. The findings will help us to design and develop new materials with improved functionality and durability. Another area of interest is to apply this method to the metal-biological interface to understand the degradation of metallic implants and how different alloying elements influence new bone formation. Our ultimate goal would be to develop implant materials with excellent biodegradability and biocompatibility and to understand the biomineralization process on a near-atomic scale. The group will work closely with the Atom Probe Tomography, Microscopy and Diffraction, Transmission Electron Microscopy, Nanoanalytics and Interfaces, Computational Electrochemistry and Corrosion groups to improve our understanding of interfacial reactions/processes at the liquid-solid interface and the influence of microstructure and different dissolved ions in electrolytes on the corrosion process. The group will also work closely with collaborates from external research institutions at ETH Zurich on the corrosion of iron-based materials. These collaborations will enable us to gain a better fundamental understanding of interfacial processes at the liquid-solid interface to slow down or prevent the degradation of materials and thus develop better materials for a more sustainable future.