The "Electrochemistry and Corrosion" group
The aim of the group "Electrochemistry and Corrosion" is to develop and apply ab-initio based mutli-scale simulation techniques to tackle problems in electrochemistry, with a strong focus on corrosion and related topics.
The various activities in the group related to
- interactions of atoms and molecules with surfaces
- investigation of materials' stability and understanding of stabilisation/destabilisation mechanisms
- identification of stable and metastable surface/interface structure subject to the given environmental conditions
- understanding degradation mechanisms
- properties of ions in water and computation of accurate formation energies of ions in water
are tailored to provide in-depth insight into the functionality and evolvement of materials properties under the influence of the environment. Such insight will aid the development of strategies to counteract, retard or suppress degradation phenomena in materials.
Method development and application
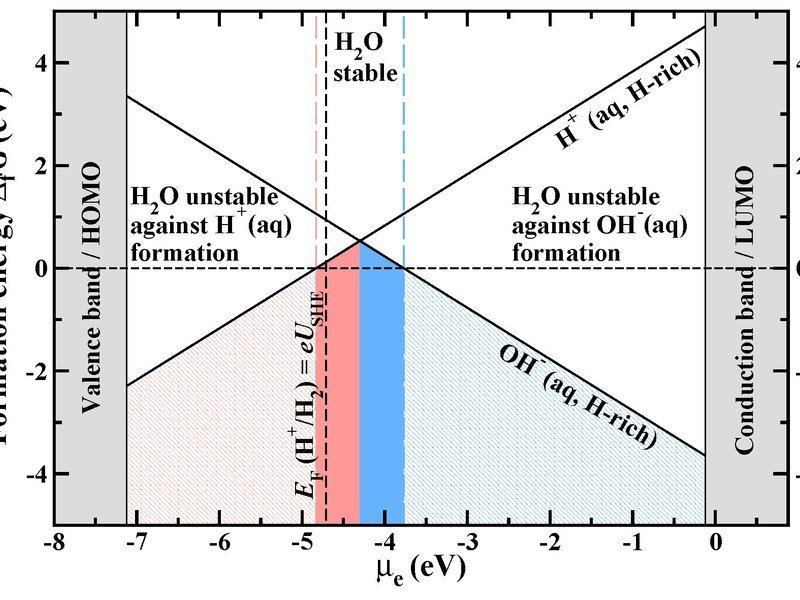
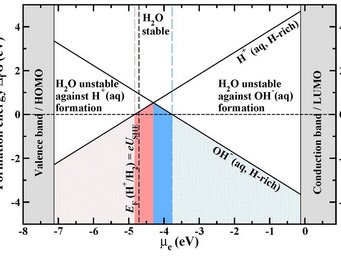
Formation energy of the native water ions in water as a function of the electron chemical potential at hydrogen rich conditions. The zero point of the Fermi energy
corresponds to the vacuum level. The vertical dashed black line marks the position of the standard hydrogen
electrode (SHE). Regions of H+(aq) and OH-(aq)) ion dominance are shown in red and blue.
corresponds to the vacuum level. The vertical dashed black line marks the position of the standard hydrogen
electrode (SHE). Regions of H+(aq) and OH-(aq)) ion dominance are shown in red and blue.
© Mira Todorova, Max-Planck-Institut für Eisenforschung
- We have derived a unified approach that is based on a fully grand-canonical description of both ions and electrons and that connects and “translates” concepts in semiconductor defect chemistry and electrochemistry. Employing this approach provides surprising new insight into apparently ”old” problems such as water stability, opens new routes to construct electrochemical phase (Pourbaix) diagrams, and gives a handle to an absolute alignment of electrochemical potentials. [1]
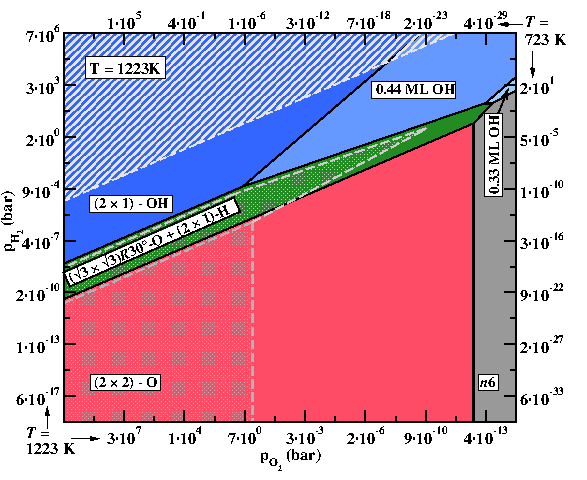
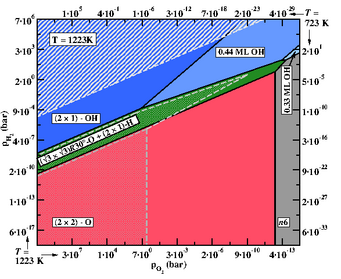
Temperature dependent phase diagramme for ZnO(0001)-Zn surface for T = 950°C (filled areas). The dot-filled areas depict how the boundaries for two of the phases change as the temperature is lowered to T = 450°C.
© Mira Todorova, Max-Planck-Institut für Eisenforschung
- Extending the concept of equilibrium surface phase diagrams to describe kinetically stabilised surface reconstructions by constructing meta-stable phase diagrams and going beyond the thermodynamic limit enabled us to account for non-equilibrium conditions, despite the absence of explicit kinetic modelling. We also demonstrated that the common belief that temperature effects are restricted to the T-dependence of the gas phase chemical potentials is not always justified and that there are materials for which the surface vibrational entropy has an unexpectedly large impact on the stabilisation of surface phases. [6,7]
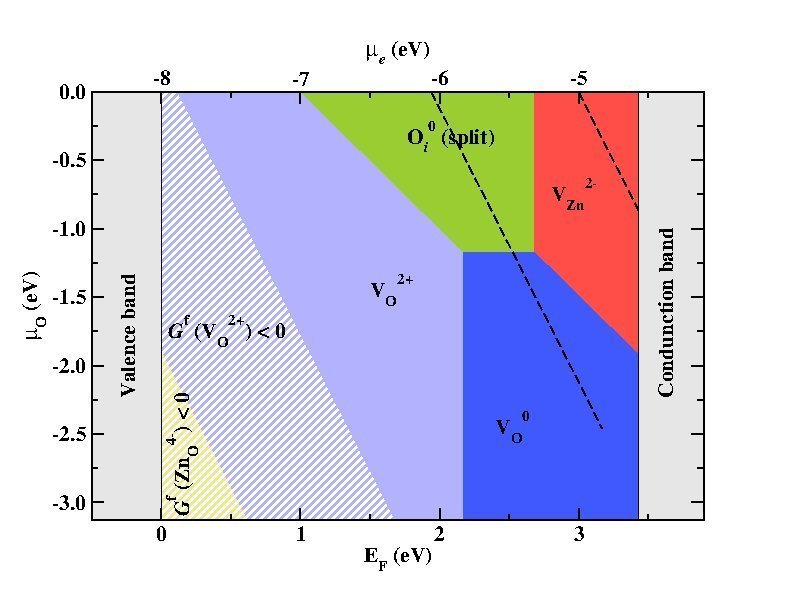
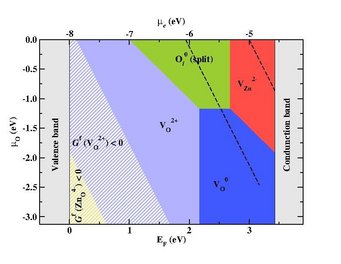
Defect stability phase diagram for ZnO showing areas of dominance of native point defects (filled areas) and areas where ZnO becomes thermodynamically unstable against the formation of its native point defects (striped regions) with superimposed electrochemical stability window of water (black dashed-lines).
© Mira Todorova, Max-Planck-Institut für Eisenforschung
- Defect phase diagrams which show the majority defect species as function of applied bias and chemical potential allow us to discuss the impact an aqueous electrolyte can have on the defect chemistry and thus on the electronic structure of a semiconducting electrode, to identify areas of interest in the context of electrochemical applications and to specify which point defects within the bulk of the semiconducting material will be influenced by the electrochemical environment, impact the properties of the solid and be relevant in the context of corrosion.[5]
People |
Projects |
Publications |