Microstructure and Interfaces of Battery Materials
The group aims at unraveling the inner workings of ion batteries, with a focus on probing the microstructural and interfacial character of electrodes and electrolytes that control ionic transport and insertion into the electrode.
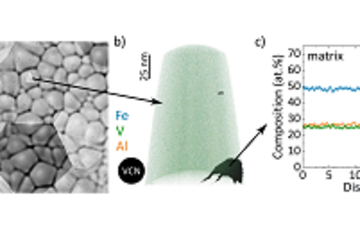
The microstructure of a material describes the distribution and arrangement of e.g., grains, pores, defects, and interfaces. It strongly influences the physical properties of materials, including strength, toughness, ductility, hardness, along with its corrosion resistance.
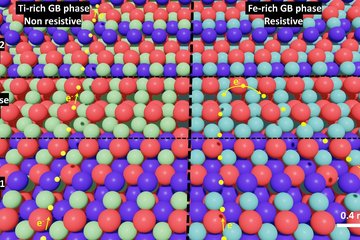
In batteries, the microstructure of the electrodes and electrolytes plays a crucial role in determining their performance and degradation. For example, the size, shape and distribution of the active particles can affect the rate of charge and discharge, the capacity retention and the safety of the battery. The porosity and tortuosity of the electrode can influence the transport of ions and electrons, as well as the mechanical stability and thermal management of the battery. The interfaces between different materials can introduce additional barriers or pathways for ion transport, depending on their chemistry and morphology.
Therefore, understanding and controlling the microstructure of battery materials is essential to improve their functionality and durability. The group’s aim is to use diverse techniques to synthesize, characterize and model the microstructure of battery materials at different length scales. This experimental group is complemented by Computational Energy Storage Material’s group and will work closely with other groups including Atom Probe Tomography, Mechanics at Chemical Interfaces, Sustainable synthesis of Materials and Corrosion. Such close and interdisciplinary collaboration enables chemical, microstructural, interfacial, and mechanical characterization of battery materials in-operation and/or ex-situ conditions. Some key areas of research for the group include are:
- microstructural effects on ion-kinetics in the electrode and the electrolytes.
- Grid storage of energy (Iron-Air battery)
- Solid-state batteries (lithium and sodium).
- mechanical behavior of battery materials.
- Interfacial reaction during SEI formation.
- Lithium/Sodium plating for anode free batteries.