Iron powder as metal fuel
To successfully transition from fossil fuels to a sustainable carbon-free energy supply, a safe and stable energy storage technology is required. Recently, metallic powders, and particularly iron powder, have been proposed as a high energy density, easily storable, and commonly traded fuel. Energy production is obtained through the heat of oxidation, and the combusted products can then be reduced at the solid-state using hydrogen coming from sustainable energy sources, resulting in a complete CO2-free energy cycle. While the combustion of iron powders seems very promising in this regard, hardly anything is known about its in-process morphological, microstructural, and chemical evolution, which are critical for the circularity of the concept and the subsequent reduction process.
In this project, we develop a materials-science-based understanding of the fundamental metallurgical processes involved during the combustion of iron powders. To do so, the different phase transformations, morphological transformations as well as chemical evolution of the powder after combustion are studied for different process parameters. Iron powders combusted at the lab and industrial scales in air and with the assistance of a propane pilot flame are analyzed and compared.
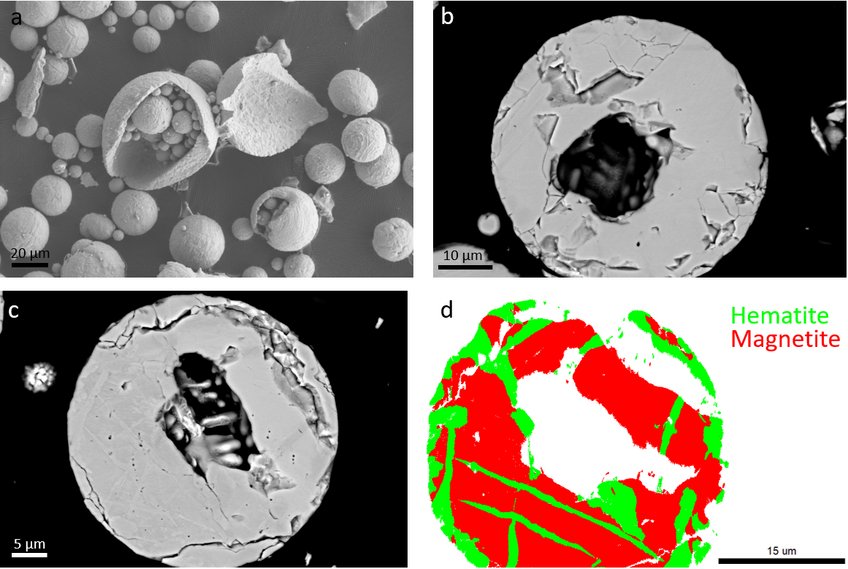
As the final phase and porosity distribution play a crucial role in the following reduction process, we compare the different morphologies and microstructures of the iron oxide powders obtained after combustion. Results (Figure 1) show that the spherical, hollow powders are obtained, with a matrix composed of magnetite and hematite laths. Many powders are open due to micro explosion events, and a relatively large crack density is obtained in the final powders.