Hydrogen plasma-based reduction of iron ores
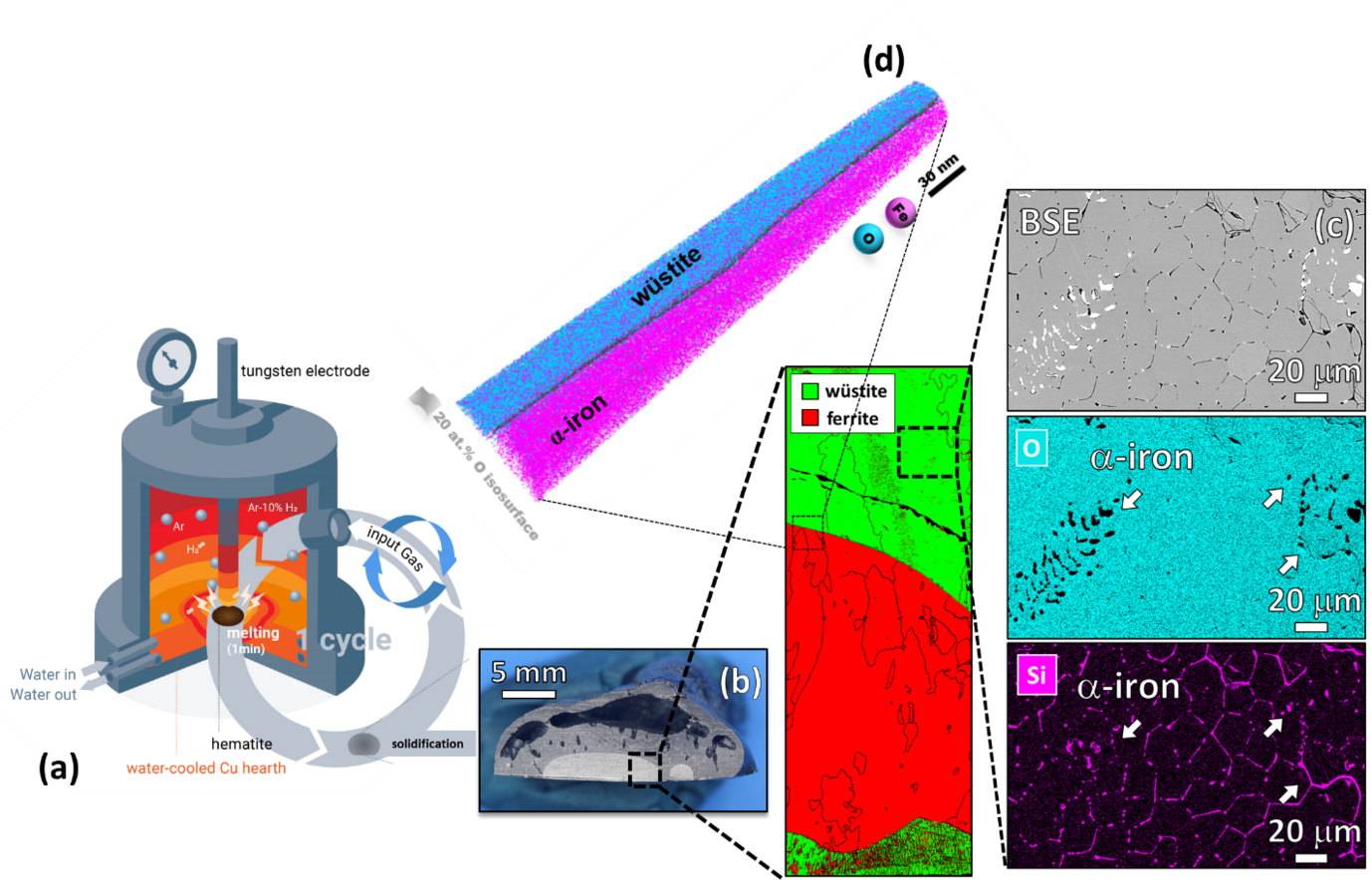
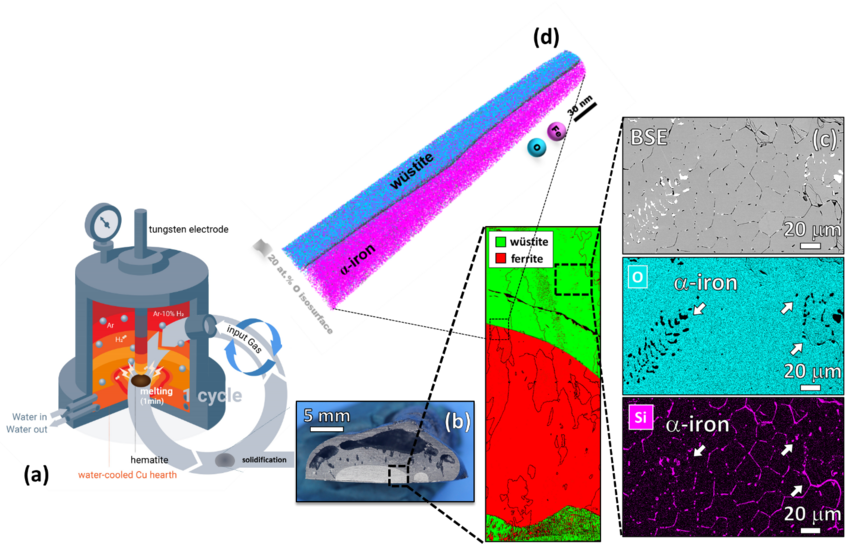
Iron- and steelmaking is the most staggering single source of CO2 emissions on the planet, accounting for ~7% of the global emissions. This fact challenges the current technologies to achieve carbon-lean steel production and reduce CO2 emissions by 80% until 2050. Among the sustainable alternatives for ironmaking, the hydrogen plasma reduction (HPR) is a promising route, as the associated by-product is water. In this process, a hydrogen plasma arc is ignited between an electrode and the ore in a conventional electrical arc furnace (EAF), Figure 1 (a). Thus, melting and reduction occur simultaneously, enabling the production of liquid iron in single step. The highly energetic hydrogen species existing in a reducing plasma also enable exothermic redox chemical reactions with enhanced kinetics, permitting energy savings.
The goals of this research project are to reveal the underlying mechanisms that drive the reduction of iron ores (e.g. hematite) via hydrogen plasma technology. Our studies focus on the reaction thermodynamics, kinetics, associated phase transformations, microstructural evolutions, and transport phenomena across several length and time scales, down to the atomistic and electronic mechanisms associated with these redox reactions. We are also particularly interested in understanding the physical and chemical interaction between hydrogen plasma arc and molten materials as well as the influence of chemistry partitioning between iron oxides and gangue-related tramp elements on the iron conversion rates.
Our first findings show that the gangue elements (especially Si) are preferentially partitioned to the unreduced oxide domains at micro and nanoscale at intermediate reduction levels, Figures 1 (c) and (d) . However, these elements get evaporated from the material over the course of the reduction process due to their higher vapor pressure compared with that of iron. Iron nearly free of gangue elements, especially S and P, was obtained after 15 min of exposure to a lean hydrogen plasma (Ar-10% H2) in a conventional lab-scale arc melt furnace without the need for expensive and time-consuming secondary metallurgy operations for removing impurities.