Hydride formation and deformation mechanisms in titanium
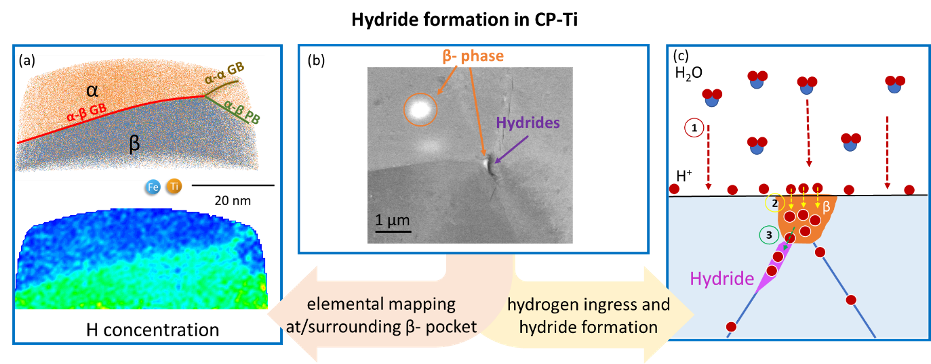
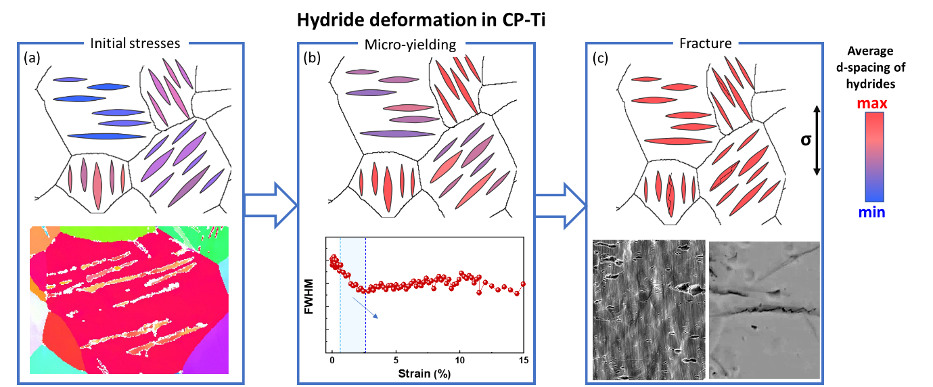
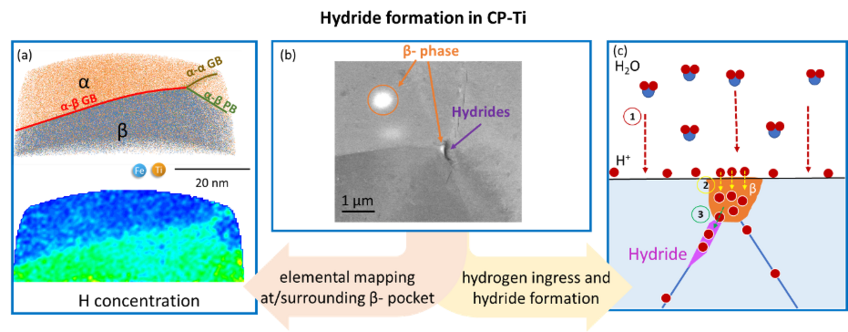
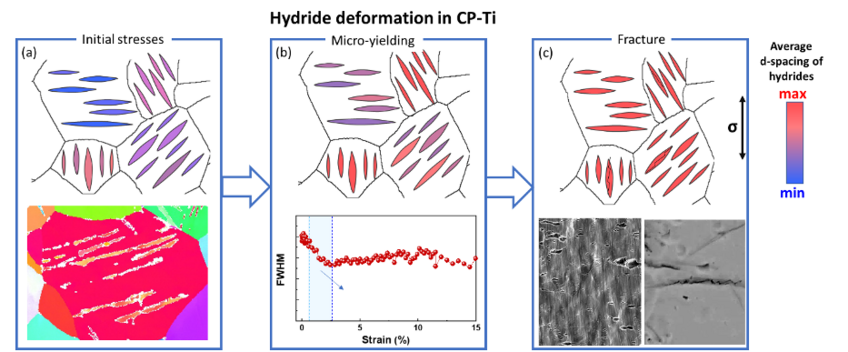
This project targets hydrogen behaviour and hydride formation mechanisms in commercially pure titanium (CP-Ti). A particular focus is on the role of β-pockets. Additionally, knowledge on the deformation behaviour of hydrides and their interaction with the parent Ti matrix can help with design approaches to alleviate hydrogen embrittlement of these alloys.
Titanium and its alloys possess excellent strength-to-weight ratio and corrosion resistance making them highly desirable structural materials. Hexagonal close-packed (hcp) ⍺-Ti alloys are also among the most corrosion resistant structural materials available for application in aggressive environments. In these alloys, a sufficiently-high trace level of β-stabilizing elements can lead to the retention of a low volume fraction of β-phase, in the form of small β pockets that are in the range of 100 nm and typically located at triple points across the microstructure. The hydrogen solubility in β-Ti is ~200x higher than in ⍺-Ti at room temperature, which can cause the hydrogen concentrations in α phase to possibly quickly become sufficiently high to cause hydride formation. We use cryogenic sample preparation via focused ion beam (FIB) for atom probe tomography (APT) to analyse phase and grain boundaries near β-pockets. Preliminary findings show that Fe and H are enriched across β-pockets and at the grain boundary but no segregation was seen at the α-β phase boundary. We propose that β-stabilizing impurities have an indirect effect on the hydrogen embrittlement as they stabilize the β-pockets, which along with the grain boundaries are the key factors for the formation of hydrides. In addition, we study the deformation behaviour of the formed hydrides and their interaction with the parent Ti matrix. This work aims to bridge the atomic-scale formation and macro-scale deformation of hydrides to understand the hydrogen embrittlement of CP-Ti.