Growth of Mg-aluminate spinel at MgO- Al2O3 contacts: experiment, nature, and some theory
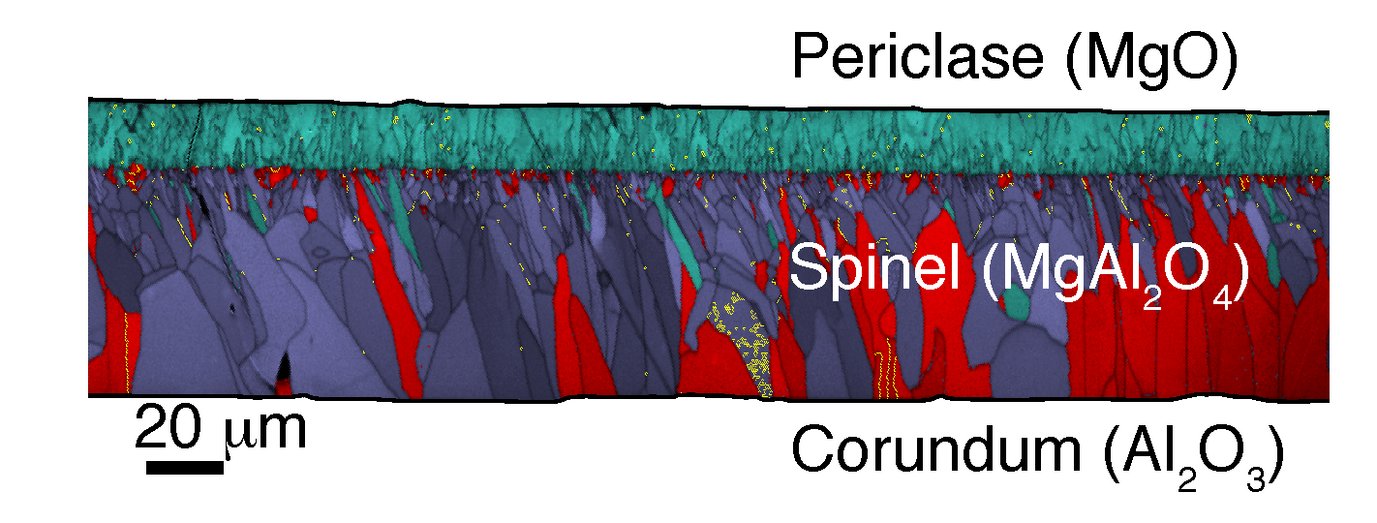
In this presentation the formation of spinel (MgAl2O4) by reaction between periclase (MgO) and corundum (Al2O3) is addressed. The reaction MgO + Al2O3 => MgAl2O4 may be regarded as a model case for diffusive phase transformations in oxide systems. All phases involved are moderately to highly refractory and have applications in ceramics. Above about 800°C, periclase and corundum react to form a layer of polycrystalline spinel at their interface. A pronounced dichotomy of the internal microstructure and texture of the spinel layer reveals the original position of the periclase-corundum interface. This reflects the direction and extent of the necessary Mg2+ and Al3+ transfer across the spinel layer and allows to quantify the underlying diffusion process. Systematic deviations of the Mg/Al ratio of the spinel from local equilibrium values at the spinel-periclase and the spinel-corundum interface are due to a finite mobility of the two reaction interfaces. The resistance against interface motion arises from dislocation climb at the periclase-spinel interface, which is complemented by the formation of Schottky defects in the reactant periclase. In contrast, the corundum-spinel interface moves by the glide of partial dislocations. This is energetically less expensive than the dislocation climb at the periclase-spinel interface and allows for comparatively rapid approximation of local equilibrium.