Electrochemical and corrosion behavior of high-entropy alloys
In this project, the electrochemical and corrosion behavior of high entropy alloys (HEAs) have been investigated by combining a micro-electrochemical scanning flow cell (SFC) and an inductively coupled plasma mass spectroscopy (ICP-MS) element analysis. The passivation behavior and composition of the passive film of the HEA has also been studied by electrochemical impedance spectroscopy (EIS) and X-ray photoelectron spectroscopy (XPS), respectively. Our main aim is to identify the electrochemical and corrosion mechanisms of the HEAs under various conditions.
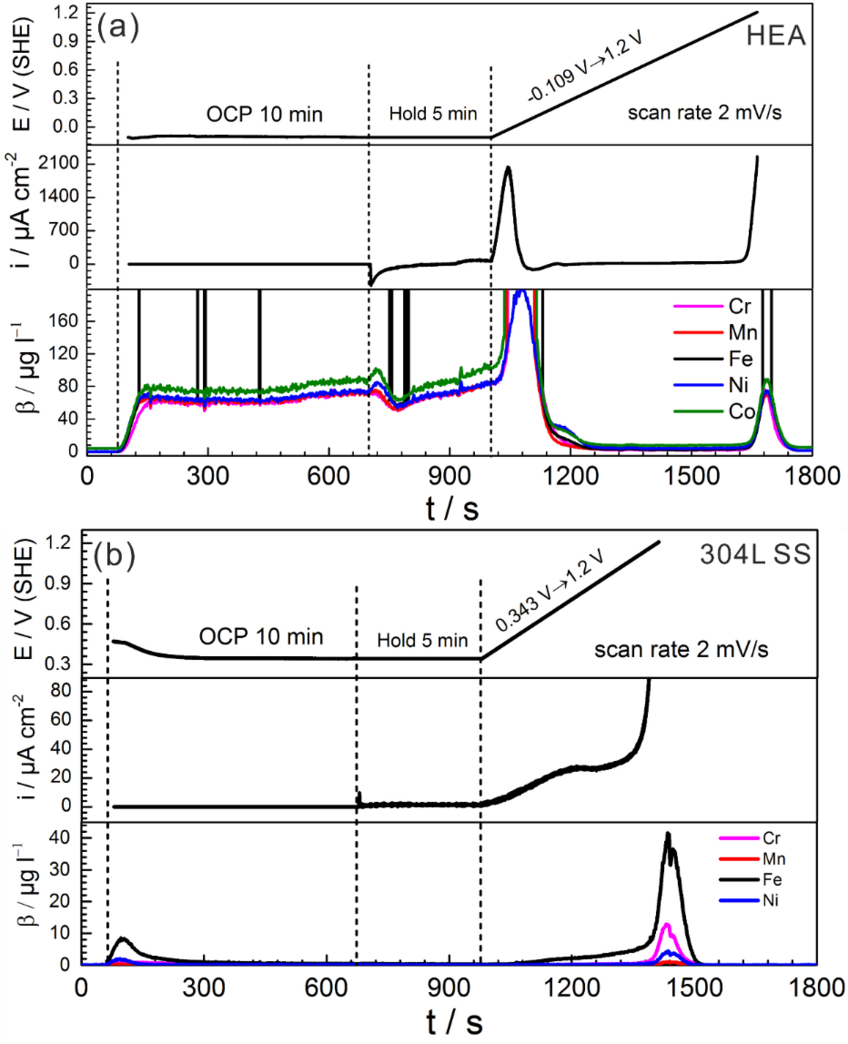
High entropy alloys (HEAs) represent a unique class of metal alloys, comprising nominally five or more elements in near equiatomic proportions. HEAs have gained significant interest on the basis that the high configurational entropy of such alloy systems is purported to result in a single-phase solid solution structure. The corrosion resistance and passive film properties of high-entropy alloys (HEAs) in various conditions were investigated. In this project, we investigated the corrosion behavior of the equiatomic CoCrFeMnNi HEA compared with the 304L stainless steel in diluted sulfuric acid solution by combining a micro-electrochemical scanning flow cell (SFC) and an inductively coupled plasma mass spectroscopy (ICP-MS) element analysis. The passivation behavior and composition of the passive film of the equiatomic CoCrFeMnNi HEA were also investigated by electrochemical impedance spectroscopy (EIS) and X-ray photoelectron spectroscopy (XPS), respectively. Moreover, the electrochemical and corrosion behavior of other types of HEAs are being investigated.
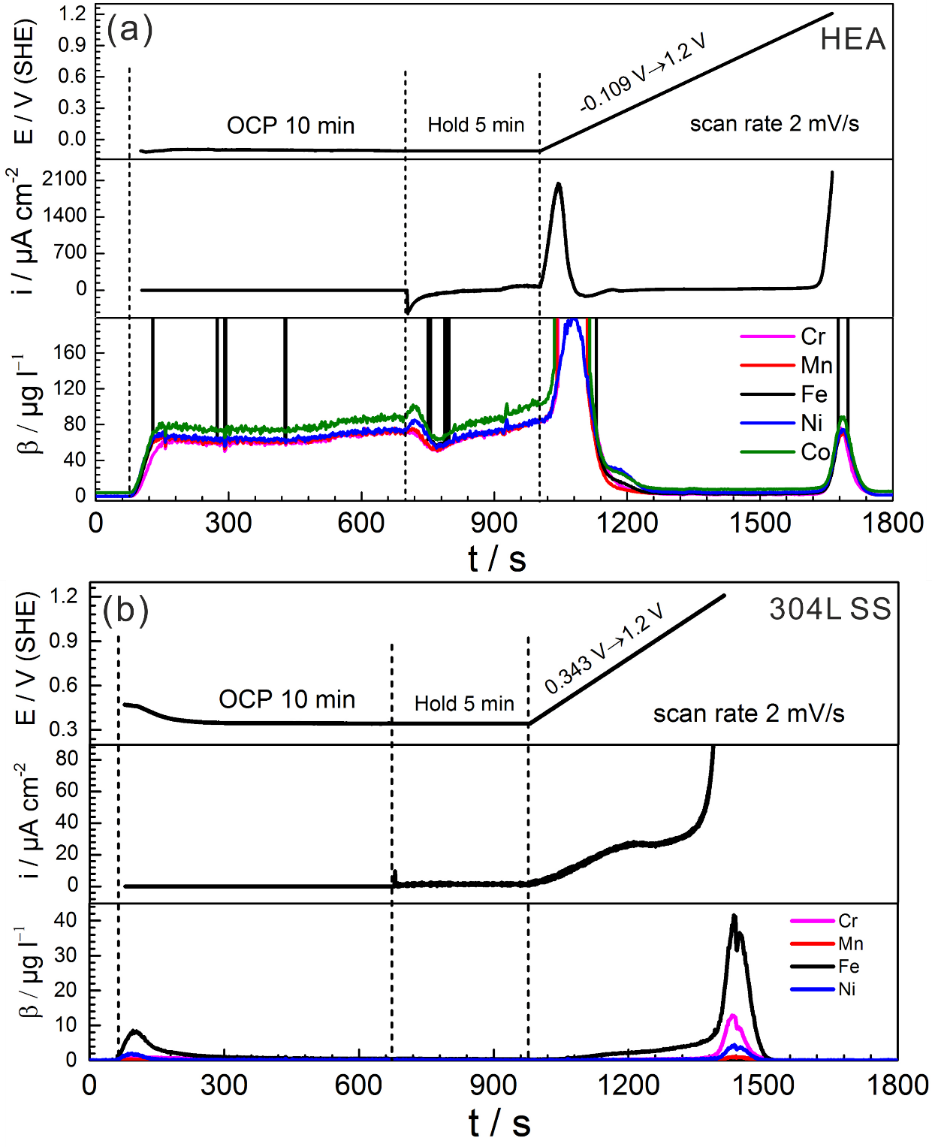
Electrochemical behavior and online in-situ element-resolved analysis in 0.1 M H2SO4. (a) Equiatomic CoCrFeMnNi HEA. (b) 304 L SS.