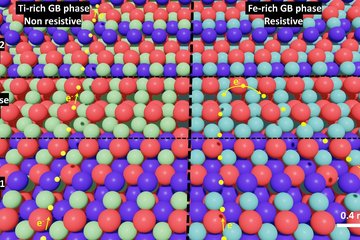
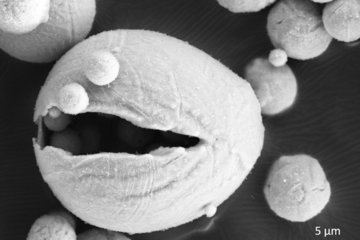
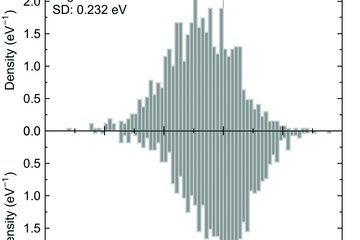
All genres
1.
Journal Article
Phase equilibria in the Fe–Rh–Ti system II. CVM Calculations. Intermetallics 15 (9), pp. 1248 - 1256 (2007)
2.
Journal Article
Phase equilibria in the Fe–Re–Ti system I. Experimental results. Intermetallics 15, pp. 1237 - 1247 (2007)
3.
Journal Article
Experimental study and Cluster Variation modelling of the A2/B2 equilibria at the Ti-rich side of the Ti–Fe system. Zeitschrift für Metallkunde 95 (6), pp. 464 - 468 (2004)
4.
Journal Article
Prototype Calculations of B2 Miscibility Gaps in Ternary B.C.C. Systems with Strong Ordering Tendencies. Intermetallics 11, pp. 1245 - 1252 (2003)
5.
Talk
CVM calculations in the bcc Fe–Rh–Ti system. Calphad XXXIV – International Conference on Phase Diagram Calculations and Associated Subjects, Maastricht, The Netherlands (2005)
6.
Talk
Design of ferritic/martensitic heat resistant 650 °C steels supported by thermodynamic modelling. DGM, Werkstoffwoche 2004, München, Germany (2004)
7.
Talk
Experimental study and thermodynamic modelling of the Fe-Ta equilibrium phase diagram. TOFA, Discussion Meeting on Thermodynamics of Alloys, Wien, Austria (2004)
8.
Talk
The modelling of important intermetallic phases, existing in Fe-based systems by the combined CALPHAD and ab-initio approach. CALPHAD XXXIII, Krakow, Poland (2004)
9.
Talk
Phase Equilibria in the Ternary Fe–Rh–Ti System. TMS Annual Meeting 2003, International Symposium on Intermetallic and Advanced Metallic Materials – A Symposium dedicated to Dr. C.T. Liu, San Diego, CA, USA (2003)
10.
Poster
Thermodynamic assessment of Laves phase and µ-phase in Ta–X–V systems. CALPHAD XXXII, La Malbaie, Québec, Canada (2003)
11.
Thesis - PhD
Phase Equilibria in Fe–Rh–X (X=Ti,Co) Systems. Dissertation, RWTH, Aachen, Germany (2004)