Climate-friendly metals from deep-sea ores
If manganese nodules can be mined in an environmentally friendly way, the critical metals needed for the energy transition could be produced with low CO2 emissions
At a glance:
- Global demand for copper, nickel and cobalt is expected to more than double by 2050. Producing these metals from deep-sea manganese nodules would, among other things, avoid deforestation and generate substantially less waste.
- A Max Planck team extracts elemental copper from deep-sea ore by simply melting it in an electric-arc furnace. Using hydrogen plasma as the reducing agent then creates an alloy that contains, among other elements, nickel and cobalt.
- The process emits 90 per cent less CO2 and requires almost a fifth less energy than the conventional coal-based reduction method.

The demand for metals will increase significantly in the coming years, primarily because the climate-friendly transformation of the economy is only possible through the electrification of industrial processes, transport and heat generation. By 2050, around 60 million tonnes of copper will be needed for electric motors and the expansion of the electricity grid. Moreover, depending on how battery technology develops, a further 10 million tonnes of nickel and 1.4 million tonnes of cobalt may also be required. Demand for copper and nickel would therefore more than double by the middle of the century, while demand for cobalt could increase fivefold. The extraction of metals always has a negative impact on the environment. Large areas of forest are repeatedly cleared for nickel and cobalt mining. And the mining of cobalt in particular often takes place under very questionable social conditions: according to UNICEF, children are often sent to work in the mines. The ores found on land also contain only a very small proportion of the metals sought. For every tonne of copper mined from deposits on land, some 200 tonnes of waste are produced, and taken together, the annual production of copper, nickel and cobalt generates between 4 and 5 billion tonnes of unusable rock and slag.
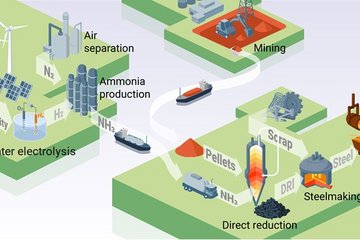
An alternative to land-based mining is the extraction of deep-sea ore nodules, commonly known as manganese nodules, which, besides large amounts of manganese, also contain a significant proportion of copper, nickel and cobalt. They are found in large quantities in the Clarion-Clipperton Zone in the Pacific. A team from the Max Planck Institute for Sustainable Materials has now presented an efficient and low-CO2 process in the journal Science Advances in which copper, nickel and cobalt can be extracted from deep-sea ore by smelting and reducing it with hydrogen. The method is significantly more sustainable than the process used by the Canadian company TMC for the reduction of deep-sea ores with carbon-based compounds in its Nori-D project. Using hydrogen for reduction cuts CO2 emissions by more than 90 per cent if green hydrogen and renewable electricity are used. The Max Planck team’s approach also requires almost 20 per cent less energy and fewer process steps.
No deforestation and significantly less waste in deep-sea mining

“The extraction of these nodules in the deep sea also leaves an environmental footprint,” says Dierk Raabe, Director at the Max Planck Institute for Sustainable Materials. “That's why I was against exploiting these resources just a few years ago, so we wouldn’t repeat the same mistakes we made on land.” In the meantime, however, the materials scientist has become open to the idea of deep-sea mining – at least if it is carried out in the most environmentally responsible way possible. His view has changed for several reasons, not least because the extraction of metals from deep-sea ores would not involve child labour and would result in far less deforestation and far less waste. For example, the production of the metals for 1 billion electric car batteries would generate 9 billion tonnes of rock waste if the materials were extracted from deep-sea ores, while 63 billion tonnes of unusable rock would have to be dumped if they were extracted from deposits on land. This is what researchers at the University of Delaware have calculated.

The environmental footprint of metal production from deep-sea ores would therefore be significantly smaller using the Max Planck team’s process. “We reduce the dried ores with a hydrogen plasma directly in an electrically operated arc furnace,” explains Ubaid Manzoor, who carried out the experiments as part of his doctoral research. The researchers are already able to recover almost all of the copper as pure metal by melting the ore and then allowing the molten metal to cool slightly. As soon as they allow hydrogen to flow into the furnace, an alloy of copper, nickel and cobalt, among other elements, is produced alongside various manganese oxides, some of which can be used in batteries. The proportions in the alloy vary depending on the duration of the reduction. “Because we can separate copper first, it becomes easier to process the remaining alloy,” explains Manzoor. In an earlier study, Manzoor and his team of researchers had already presented a very similar process that can be used to extract nickel from ores mined on land in a climate-friendly way.
A contribution to the comprehensive life cycle assessment of deep-sea mining
Whether deep-sea mining will one day replace land-based mining remains the subject of international negotiations. “With our work, we want to provide a sustainable method for extracting critical metals from deep-sea nodules and the data needed for informed decisions, considering the environmental impacts from both mining of the ores and processing of the ores”, says Manzoor. And for Raabe, one thing is clear: “If we want to move away from a CO2-intensive economy, we will have to accept some bitter pills.”
Author: Peter Hergersberg, Max Planck Society