Metal powder: zero-carbon fuel for the future?
How iron can be used to store and transport energy
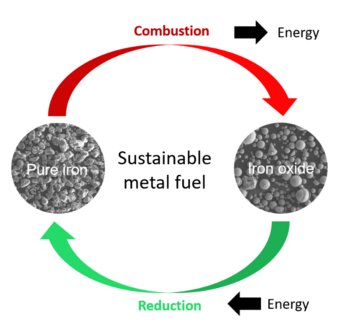
Energy from sun or wind is weather-dependent and lacks an efficient way to store and transport it. Scientists from the Max-Planck-Institut für Eisenforschung and TU Eindhoven are investigating iron as a possible energy carrier. The idea is to store excess energy in iron and release it through combustion of iron into iron oxide. The team is working to understand the underlying processes and upscale the technique to industrial relevance.
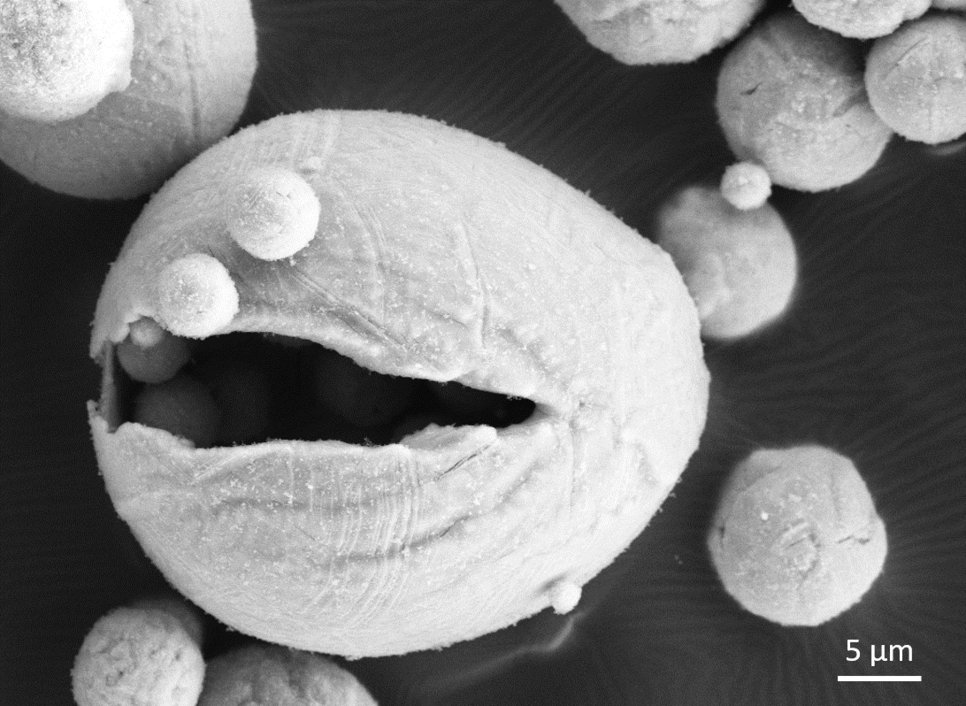
Gaining sustainable energy from wind, solar and water is commonly known and applied. However, renewable sources depend on environmental conditions: in peak times of wind and sun, excess energy is produced that is needed in times of less wind and sunshine. But how to store and transport this excess energy efficiently? So far, no reliable, safe and cheap way has been found to store a high amount of energy in a small volume container. Now, scientists from the Max-Planck-Institut für Eisenforschung (MPIE) and the Eindhoven University of Technology analysed how metals, particularly iron, can be used for energy storage and which parameters determine the efficiency of the storage and reuse. They published their recent findings in the journal Acta Materialia.
Creating a circular reduction and combustion process
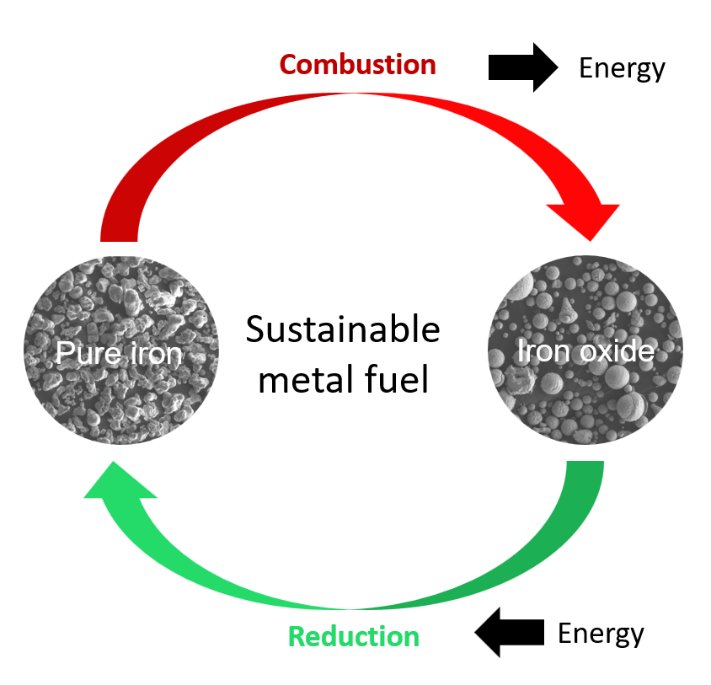
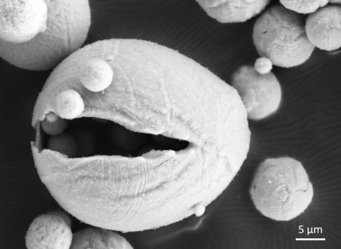
“Storing energy in metals and burning them to free the energy whenever needed is a method already applied in aerospace technology. Our aim was to understand what exactly happens at the micro- and nanoscale during the reduction and combustion of iron and how the microstructure evolution influences the efficiency of the process. Additionally, we wanted to find how to make this process circular and keep energetical losses as low as possible”, explains Dr. Laurine Choisez, who recently finished her postdoctoral research at the Max-Planck-Institut für EIsenforschung and who is first author of the publication. When iron ores are reduced to iron, a lot of energy is naturally stored in the reduced iron. The idea is to get this energy out of the iron whenever needed by oxidizing the iron back to iron oxide. In times of excess energy from wind, sun or water, this iron ore could be again reduced to iron and the energy stored. The scientists speak of combustion when describing the “burning”, meaning oxidation, of the iron back to iron ore. Choisez and her colleagues at Max-Planck-Institut für Eisenforschung focussed on the characterization of the iron powders after reduction and combustion using advanced microscopy and simulation methods to analyse the powder purity, morphology, porosity and the thermodynamics of the combustion process. The obtained microstructure of the combusted iron powders is decisive for the efficiency of the following reduction process, and to determine whether the process of reduction and combustion is fully circular, meaning that no additional energy or material has to be added.
Upscaling for industrial use
The scientists present two combustion pathways, one supported by a propane pilot flame and one self-sustained in which the only fuel used is the iron powder, and show how the combustion pathway influences the microstructure of the combusted iron. “We are currently upscaling the reduction and combustion steps to an industrial relevant level determining the exact parameters like temperature and particle size, which are needed”, explains Niek E. van Rooij, doctoral researcher in the Combustion Technology group of the Eindhoven University of Technology and co-author of the publication. The recent study showed that using metals to store energy is feasible. Future studies will now analyse how to increase the circularity of the process, as the size of some combusted particles is decreased compared to their original size due to partial iron evaporation, micro-explosions and/or fracture of some iron oxide particles.
Author: Yasmin Ahmed Salem