Compositionally complex hydride formation in transformation-induced plasticity refractory high entropy alloys
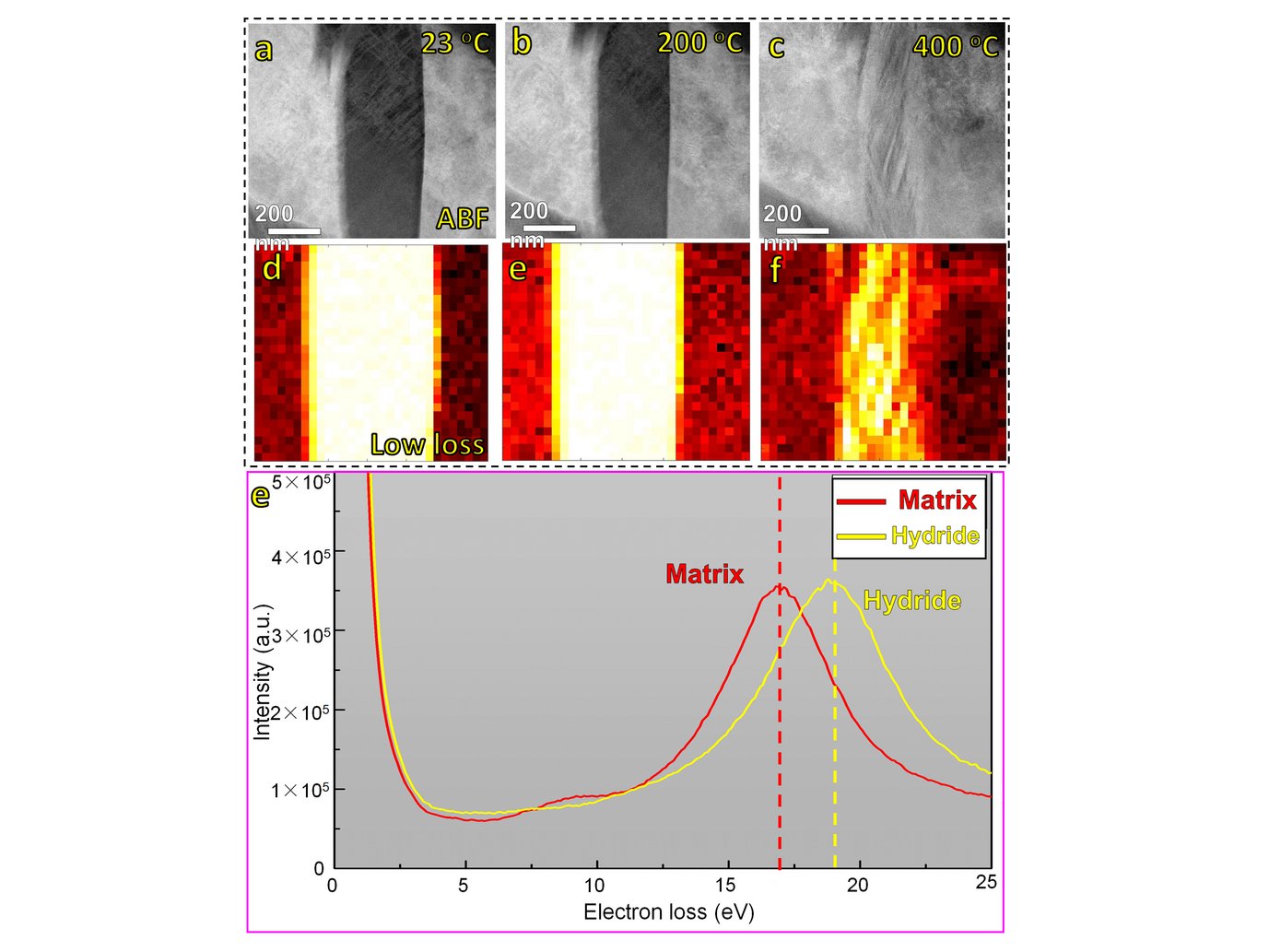
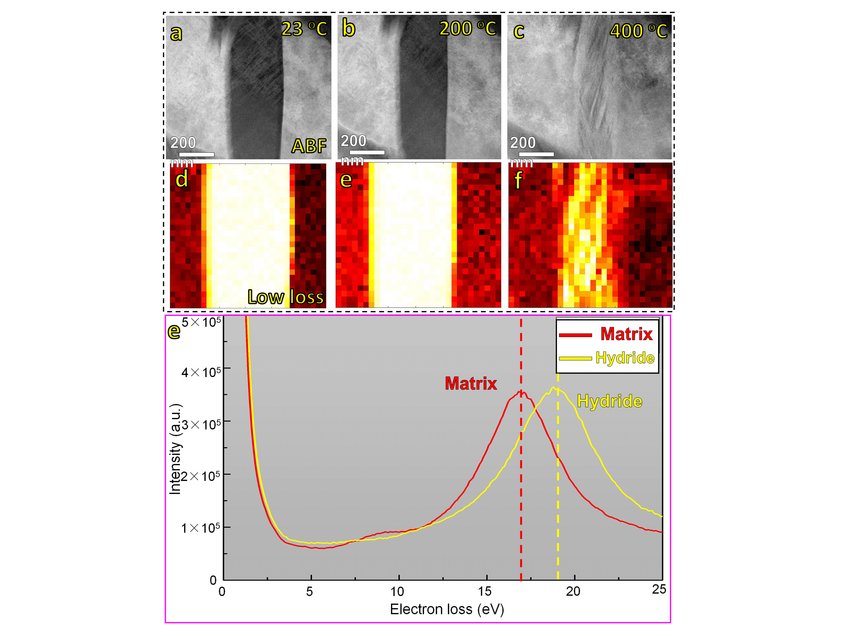
Except for their exceptional mechanical properties, high entropy alloys (HEAs) are also an attractive field of research owing to their promising functional properties that might play an important role in establishing a green energy landscape. For instance, refectory high-entropy alloys (RHEAs) containing hydride forming elements, such as Ti, Zr and Hf, exhibit good prospects as hydrogen storage materials due to the formation of hydrides with high hydrogen/metal ratio [1–4]. We are currently investigating a novel type of TRIP-induced dual-phase RHEA to design a mechanically stable material with tunable hydride formation capabilities. The HCP fraction can be simply adjusted by the amount of pre-straining at room temperature. Upon electro-chemical charging compositionally complex hydrides form from the surface of the sample into the bulk within the HCP regions. Their global morphology is characterized by electron channeling contrast imaging (ECCI) and electron backscatter diffraction (EBSD). Conventional TEM and atomic resolution imaging reveal the atomic nature of these new types of hydride and their dissolution mechanisms are observed by in situ heating experiments (Figure. 1). Here, plasmon loss EELS is used to determine the thermal stability of the hydrides, which is ~400oC in the high vacuum column of the microscope. Further heating leads to a complex transformation of the initial FCC hydride phases within the HCP back to BCC. We further use atom probe tomography (APT) to determine the concentration of metal elements in the hydride phases and the corresponding distribution of deuterium and hydrogen. These underlying mechanisms will guide the design of a material with good structural integrity and hydrogen storage capacity.