Interstitial Alloying of High Entropy Alloys
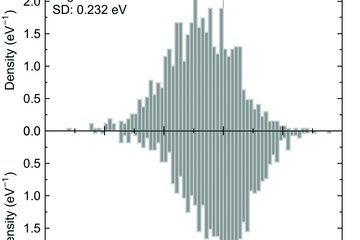
Interstitial alloying can improve the mechanical properties of high-entropy alloys (HEAs). In some cases, the interstitial-alloying impact is very different from those in conventional alloys. We investigate the effect of interstitial alloying in fcc CrMnFeCoNi HEA as well as bcc refractory HEAs, particularly focusing on the solution energies and impact on, e.g., stacking fault energies, based on first-principles calculations. Our results clarity, e.g., that the interstitial solution energy in HEAs is no longer a single value but shows a substantial distribution due to the dependence on local chemical environments.
Interstitial alloying is one of the typical approaches to improve the mechanical properties of high entropy alloys (HEAs) as well as traditional alloys. In experiments, carbon increases the ultimate strength of CrMnFeCoNi-based HEAs while keeping good ductility [1]. More interestingly, hydrogen is found to improve both the strength and the ductility of CrMnFeCoNi [2], unlike the traditional alloys that show hydrogen embrittlement. While these observations motivate us to design the mechanical properties of HEAs by interstitial alloying, from the atomistic viewpoints, little is so far known for the impact of interstitial alloying in HEAs. Particularly, in HEAs, each interstitial site has distinct local chemical environment, which is however difficult to access only by experiments.
For face-centered cubic (fcc) alloys, their ductility as well as their deformation behavior is closely connected with their stacking fault energies (SFEs), as well known for e.g. high Mn steels. For the design of mechanical properties of HEAs by interstitial solid solutions, it is therefore essential to elucidate its impact on SFEs. One of the aims of this project is therefore to explore the impact of interstitial alloying on SFEs of fcc HEAs with C and N [3,4].
More recently the possibilities of interstitially alloyed bcc high entropy alloys are explored. In the courtesy of this project the solubility and impact of different interstitials (like, e.g., C, N, or O) in bcc HEAs will be investigated.
Further reading
Other related projects on HEAs are, e.g., on short-range order and phase stability.
Acknowledgments
This work has received funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) within the special priority programme “Compositionally Complex Alloys – High Entropy Alloys (CCA-HEA)” (SPP 2006).
References
[1] Z. Li, C. C. Tasan, H. Springer, B. Gault, and D. Raabe, Sci. Rep. 7, 40704 (2017).
[2] H. Luo, Z. Li, and D. Raabe, Sci. Rep. 7, 9892 (2017).
[3] Y. Ikeda, I. Tanaka, J. Neugebauer, F. Körmann, Phys. Rev. Mat. 3, 113603 (2019).
[4] Y. Ikeda, F. Körmann, J. Phase Equilib. Diffus. 42, 551 (2021).