Understanding the Mechanism of the Oxygen Reduction Reaction on Pt
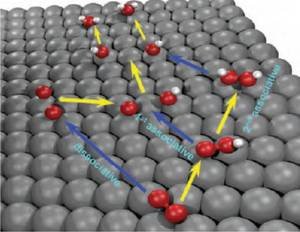
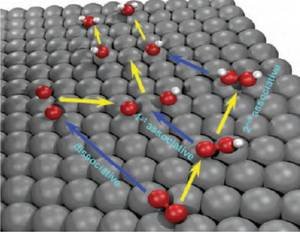
The oxygen reduction reaction (ORR) is a fundamental reaction related to various disciplines such as energy conversion, material dissolution or biology. Recently, particular interest focused on its essential role in fuel cells or lithium-air batteries. However, the mechanism of the ORR on metal surfaces remains unclear. The distinction between the ORR mechanisms is based on the number of proton-coupled electron transfer steps that precede the O–O bond breaking step (Fig. 1). Among these mechanisms, hydrogen peroxide can be formed as an intermediate of ORR only by the 2nd associative mechanism. Indeed, hydrogen peroxide has been detected under certain conditions during ORR, but it remains unclear whether it is a key intermediate of the dominant ORR mechanism or a side-product [1]. A detailed understanding of the interaction of H2O2 with metal surfaces is essential on the road to understanding the ORR mechanism.
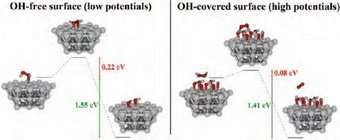
In weakly adsorbing electrolytes such as HClO4, the total rate of H2O2 decomposition on polycrystalline Pt is controlled by mass transport, in the potential region between +0.2 VRHE and +1.5 VRHE. In this region, the currents in the cyclic voltammograms (CVs) scale with the thickness of the diffusion layer and with the bulk concentration of H2O2 [2,3]. In addition, electrolysis experiments performed under potentiostatic conditions indicate that the rate of decrease of H2O2 concentration with time is diffusion-limited, regardless of the applied potential. This means that during ORR in such a system, H2O2 cannot be detected in the electrolyte even if it is formed at the interface. In a peroxide-containing solution, the potential-dependent Pt surface state triggers the corresponding reaction: Upon interaction with reduced surface atoms at low potentials, H2O2 adsorbs dissociatively producing OHads, while upon interaction with an oxidized surface at high potentials, H2O2 gets oxidized to O2 by reducing the surface [3]. The measured current is the sum of the two partial currents restoring the thermodynamically preferred surface state at a given potential. Quantum chemical ab initio calculations (Fig. 2) showed that the activation barriers for either H2O2 dissociation or oxidation are easily overcome by the thermal energy and the reactions will proceed with a high rate at room temperature [2].
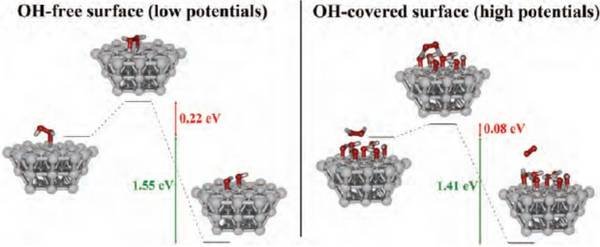
Therefore, the kinetics of both reactions of H2O2 dissociation (at low potentials) and H2O2 oxidation (at high potentials) are very fast as long as there is sufficient availability of platinum sites capable of carrying out any of the two reactions. This condition is fulfilled in weakly adsorbing electrolytes, where the coverage from inhibiting adsorbates is low. In particular, at potentials relevant to the ORR (i.e. below 0.95 VRHE) the barrierless cleavage of the O–O
bond of H2O2 is not prevented by the adsorption of inhibiting adsorbates.
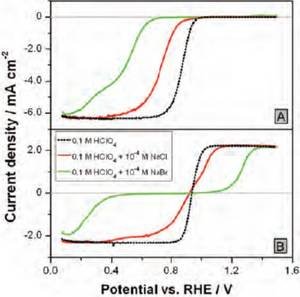
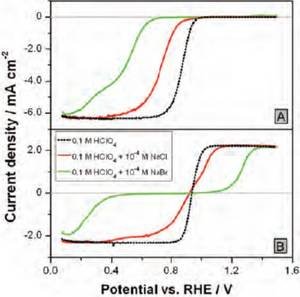
The adsorption of halide spectator species limits the availability of sites that can break the O–O bond of O2 and H2O2 [4]. The effect becomes stronger the higher the concentration of the halides in the electrolyte. Chloride adsorption inhibits the dissociative adsorption of O2 more strongly than that of H2O2 (Fig. 3), implying that a larger number of adjacent Pt atoms is required to break the O–O bond of O2, compared to H2O2. When a larger ion is added (such as bromide) the situation changes, and the inhibition of H2O2 reduction becomes stronger than that of O2 (Fig. 3). This is because the O–O bond breaking is no longer possible for both O2 and H2O2; however, there are still some single non-covered Pt atoms able to carry out the reduction O2 to H2O2 without O–O bond cleavage. Therefore, depending on the extent of the inhibition of peroxide´s O–O bond breaking, the total rate of H2O2 decomposition may not be anymore limited by the mass transport of H2O2, which translates to a local H2O2 concentration higher than zero. Thus, during ORR under such conditions, the macroscopic detection of H2O2 as an intermediate of ORR will be possible at potentials where the coverage from the inhibiting spectators is sufficiently high.
In summary, H2O2 is unstable on Pt surfaces and it will immediately dissociate to OH if formed during ORR, unless the cleavage of the O–O bond is inhibited by adsorbed spectator species. The study of the interaction of H2O2 with Pt in conditions relevant to ORR, corroborates with previously reported data on the macroscopic H2O2 formation during ORR and can explain why hydrogen peroxide has been detected under certain conditions. Therefore, the differences in the proposed ORR pathways based on experimental data are an artifact and originate only from the changes in the interface structure caused by spectators.