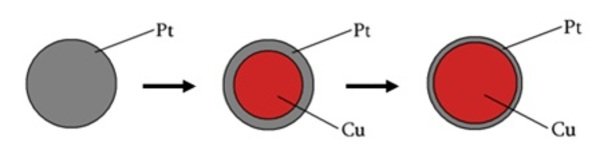
PtCu/C-Catalysts
Catalyst for PEM FC are still in a state of improving their activity. The problem of high overpotential on cathode side (oxygen reduction reaction) is still not solved.
With the use of second metal, we can make two improvements. First we can have higher electrochemical surface areas (ESA; real surface area divided by mass of platinum. m2/gPt). This is because we fill the core of a particle with a second metal and leave platinum on a surface. So the same surface area is divided by less mass of platinum.
The second improvement is the ligand effect of an alloying metal. This means that the second metal that lies under the monolayer of platinum can tune the catalytic activity of a surface atoms. So in theory it would be beneficial to make a particle of some metal and cover it with a monolayer of platinum.
In reality we have problems with the stability of such particles. The dissolution of second metal is one of the issues since it not only results in a loss of the specific activity in FC it would also poison the membrane. So it is our main concern to try to understand this degradation mechanisms in order to have right information to design more stable catalysts.