Role of Support for Activity and Stability of Fuel Cell Catalysts
As potential candidates for sustainable energy conversion systems, polymer electrolyte membrane fuel cells (PEMFC) carry high expectations in particular for mobile applications. Important problems of PEMFCs to date are a lacking practical efficiency, due to the high overpotential for the essential oxygen reduction reaction (ORR) and the high amount of noble metal catalyst in use. Degradation of the catalyst in a fuel cell during operation was identified as bottleneck for the stability of fuel cells over the last years.
Most studies on improving the stability and activity of fuel cell catalysts till now have focused on the characteristics of platinum and platinum alloy nanoparticles as electrode materials. However the support interactions between these nanoparticles and the commonly used carbon support, remain less known and understood. Therefore we are focusing on the investigation of catalyst materials with modified carbon carriers in order to gain more information about the role of the support on the activity for ORR and stability under operating conditions. These catalysts are synthesized in cooperation with the group of Prof. Ferdi Schüth at the Max-Planck-Institute for coal reseach in Mülheim.
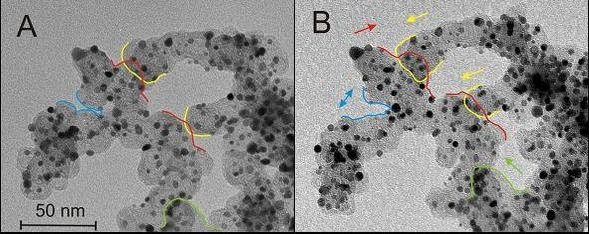
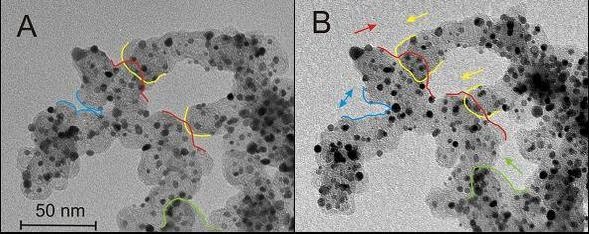
The activity of the electrocatalysts is determined employing rotating disc electrode (RDE) measurements of the limiting ORR in an electrochemical setup. The stability of the designed materials can be studied with a recently developed, non-destructive transmission electron microscopy technique. This method, called Identical Location Transmission Electron Microscopy (IL-TEM) is a powerful tool to observe changes on identical locations of a catalyst before and after electrochemical treatment ([1],[2]).