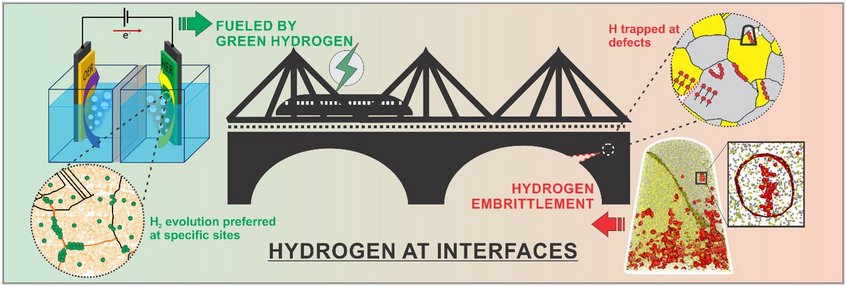
Hydrogen at Interfaces
The group studies the science of hydrogen at and across microstructural defects, both in structural and energy-related materials, in close reflection of the associated complex redox phenomena taking place in and at the surfaces of these materials. Many of these phenomena are of highest relevance for developing novel materials and processes that leverage a hydrogen-driven carbon-neutral economy, such as catalysis and sustainable material production.
In 2018, 79.5 % of the energy economy relied on non-renewable sources such as coal, natural gases, and petroleum. With rising concerns regarding climate change, the demand for cleaner and sustainable fuels is increasing. Hydrogen is the most popular candidate for clean energy for a carbon-neutral economy. On the other hand, it is known that even a few ppm of H is sufficient to cause catastrophic premature failure of materials. Therefore, to make H economy a viable solution, immediate attention is necessary to firstly, increase the efficiency of hydrogen production and secondly, provide an infrastructure where hydrogen can be used safely.
One of the topics where such delicate study setups are essential relate to the interplay of materials science and catalysis. More specifically, hydrogen production via water splitting reaction is a promising route where catalysts play a vital role in improving the energy conversion efficiency of the reaction. Many studies have indicated the role of surface composition, crystallographic orientations, facets, and microstructural defects that govern the catalytic properties. However, there is a lack of systematic investigation that deconvolutes these individual factors. By using a multiscale approach, the group focuses on understanding the catalytic performance down to sub-nm resolution. Our in-house developed reaction hub module in conjunction with analytical field ion microscopy allows us to investigate different facets and crystallographic orientations while simultaneously enabling us to probe chemical information at these regions of interest. These techniques will enable us to analyze the catalyst-hydrogen interface allowing us to reveal mechanisms that drive water splitting efficiently.
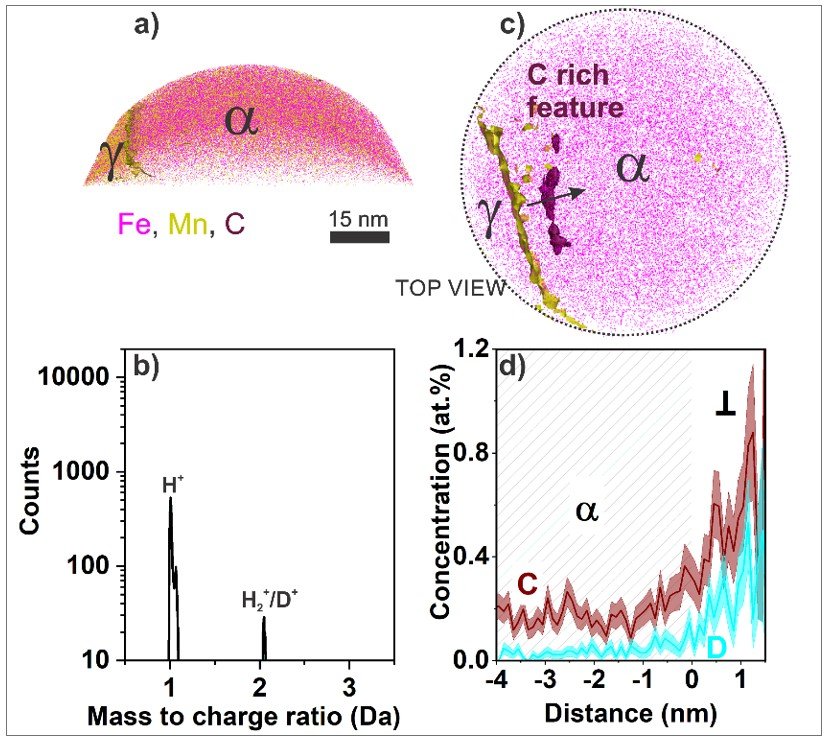
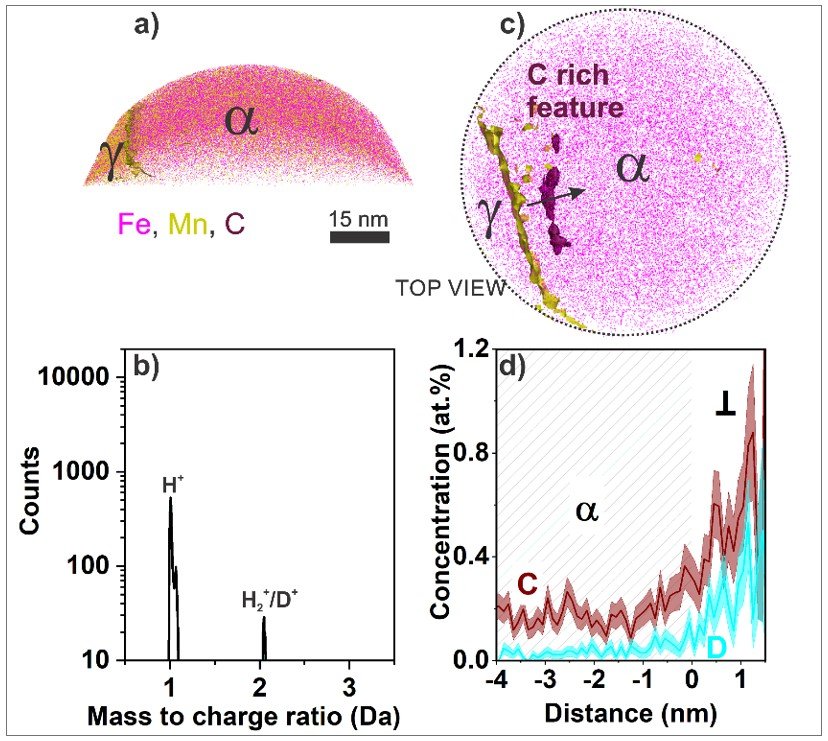
While defects are pivotal for higher catalytic activity, their interaction with hydrogen remains elusive. Many theories have been proposed to rationalize the premature failure of a material due to hydrogen embrittlement. However, one primary challenge persists which is to accurately identify hydrogen distribution inside the material. We focus on probing structural materials after hydrogen exposure under varying temperatures and pressure, at sub-nm scale. The Laplace project enabled us to freeze equilibrium states at cryogenic temperatures, hence allowing us to probe material at a vast parametric space during different stages of hydrogen exposure. Our activities thus help bridge a critical gap in the current understanding of the hydrogen-material interface.
Thus, the activities within the framework of this group, focus on understanding hydrogen production and its interaction with materials where microstructure, defects, and local/ interfacial chemistry form the basis of our investigations. The overarching aim is to design materials applied in a hydrogen-driven economy, by analyzing them at the smallest length scales after vigilantly curating their microstructure and local chemical environment. This group is envisaged to work closely with the groups of Atom Probe Tomography, Microscopy and Diffraction, Nanoanalytics and Interfaces, and Corrosion to further our understanding of the interaction of hydrogen at interfaces. These collaborative efforts will enable us to design materials that align with the ongoing pursuit of a zero-emission future.