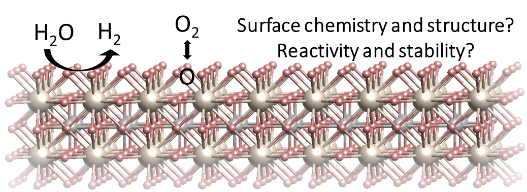
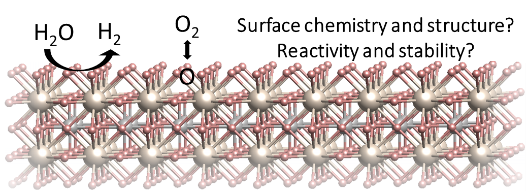
Understanding and tuning the surface chemistry of perovskite oxides to activate oxygen exchange and water splitting reactions
The deployment of decarbonization technologies, including solid oxide fuel and electrolysis cells, is limited by slow rates of conversion reactions at surfaces, and instability of materials under operating conditions. A major scientific challenge has been the lack of knowledge of the chemistry and electronic structure on material surfaces in the harsh operational conditions. Bulk transport properties of state-of-the-art perovskite type oxides in these applications are well-studied. In contrast, how the surfaces of these materials are affected by the electrochemical environment at elevated temperatures, and how these surfaces in turn affect the conversion reactions, including oxygen exchange and water splitting, have not been fully explored. Yet it is the surface that governs the reaction kinetics. In this talk, I will first discuss our research in revealing the chemical and electronic nature of surfaces in relation to oxygen exchange reactions on perovskite oxides, using in situ surface-sensitive techniques and with first-principles-based calculations. This new knowledge has guided us to design surface chemistries that eliminate a key degradation mode at the surface, and improve both activity to oxygen exchange reactions and stability. Second, I will discuss our research in understanding and controlling the formation of metal nanoparticles at perovskite oxide surfaces, to catalyze water splitting reactions. This process, called exsolution, has attracted great interest as a means to form stable metal catalyst particles on oxide supports. However, the physical factors that control the size and density of such exsolved particles are far from quantified at preset. Our research using lattice strain and ion irradiation, has shown that point defects have a key role in affecting both the thermodynamics and the kinetics of the exsolution process. Furthermore, we have revealed how the near surface region and the interior of the perovskite oxide, can undergo drastic chemical and structural changes during the metal exsolution process, with significant implications to surface reactivity as well as bulk electronic and magnetic properties. While this body of work has been pursued in the context of solid oxide fuel and electrolysis cells, the findings broadly impact also other fields that use perovskite oxides, such as in photo-electrochemical and thermochemical splitting of water, CO2 reduction, and memristive information processing.