Formation mechanisms of hydrides in environmentally sensitive materials: Ti-based alloys
Although the phenomenon was first recognized several decades ago [1], hydrogen-embrittlement remains an extremely important unsolved engineering challenge. High-performance Ti alloys are particularly prone to early fracture and reduced toughness in the presence of hydrogen or hydrides [2], which limit their possible manufacturing routes and industrial applications, leading to a risk of premature failure despite having potential for hydrogen storage [3]. The investigation of hydrogen within Ti alloys is hence essential in order to further understand the underlying mechanisms of hydrogen embrittlement and their consequences for hydrogen storage. In our work, we combined jet electro-polishing, cryogenic-focus ion beam (cryo-FIB), high angle annular dark-field scanning transmission electron microscopy (STEM), EELS and APT to show that hydride formation can originate from sample preparation, e.g., through electrochemical reactions during electro-polishing instead of the formation of a new “FCC Ti” phase (Figure 1) [4]. We further combined cryo-FIB, STEM and APT to prove that the standard site-specific FIB lift-out processes are not suitable to prepare Ti-based alloys due to the unexpected hydride formation caused by FIB milling at room temperature [5]. Lowering the temperature to -135 oC in the cryo-FIB is hence key to avoid hydride formation during sample preparation. This project has been realized in collaboration with Imperial College London in UK, RWTH Aachen and the APT group lead by Baptiste Gault in the department of Prof. Dierk Raabe (MPIE).
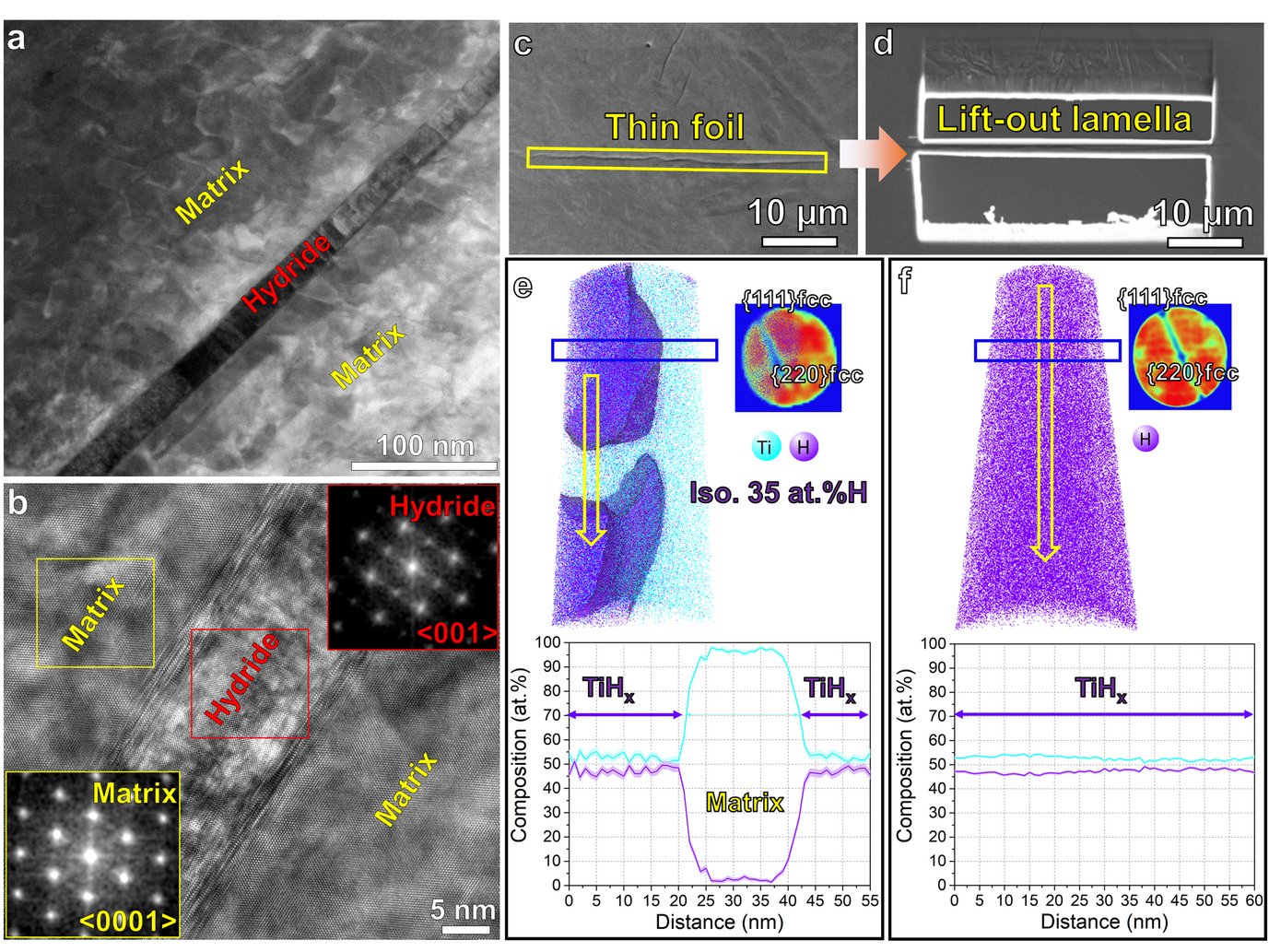
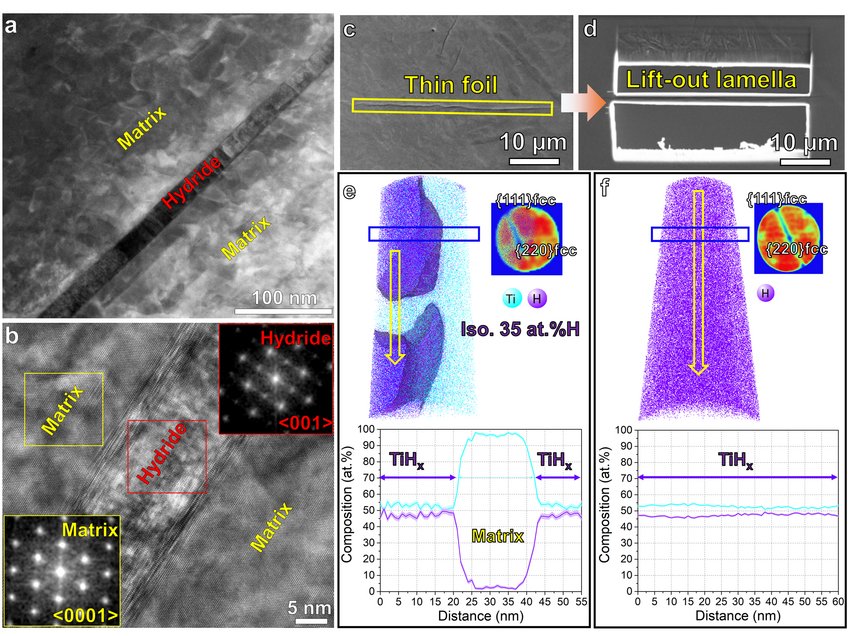
Figure 1. (a) HAADF-STEM imaging of hydride formation within Ti after jet electro-polishing. (b) HRTEM shows the orientation relationship between hydride and matrix. (c) SEM image of hydride laths in the electropolished TEM thin foil. (d) Site-specific lift-out lamella containing the long hydride lath indicated in (c); tomographic reconstruction maps of H and Ti distribution, detector histograms exhibiting the typical symmetry of FCC crystal structure, and 1D compositional profile along the yellow arrows at (e) the interface between the hydride and the α-Ti matrix, and (f) inside the hydride.