Traditional energy strategy based on employment of fossil fuels conceals two serious drawbacks, namely, amount of available fuels is limited while environmental risks during exploitation are severe. At the same time intensive research in the direction of alternative energy sources boosted general interest for concept of renewable energy. It is more than evident that one of the main challenges will be energy storage. Convenient approach is to store energy in a form of a chemical bonds where certainly attractive candidate is hydrogen. Water electrolysis as a source of very clean hydrogen, is base option to be considered.
The electrochemical decomposition of water consists of two well-known reactions: the cathodic hydrogen evolution reaction (HER) and the anodic oxygen evolution reaction (OER). It is generally accepted that platinum is the catalyst of choice for the HER, while there is still no optimal solution for the anode catalyst. Unlike in the case of the HER, where the catalyst can be considered stable during electrolysis, continuous growth of metal oxide(s) and surface reconstruction at the positive potentials during the OER induces difficulties for kinetic analysis. Moreover, fluctuation of activity over time is strongly correlated with its counterpart – stability. Despite being of crucial importance for the overall performance, however, stability is less often considered by researchers. While for a bulk electrode the loss of few monolayers is not critical, this could lead to severe degradation or even its complete loss for high surface area nanocatalysts [1].
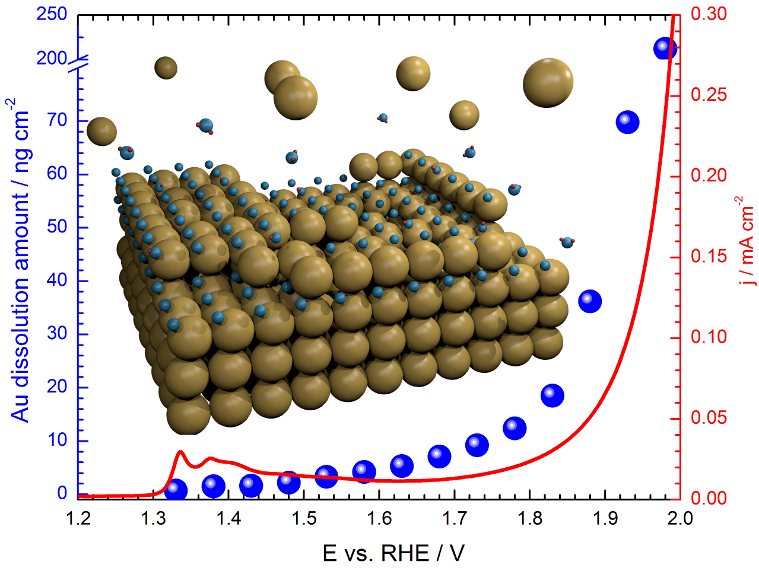
Our research is focused on the understanding of the mechanisms of noble metals dissolution (corrosion) during electrochemical processes. Recent results obtained in our group by using an electrochemical scanning flow cell (SFC) coupled with inductively coupled plasma mass spectrometer (ICP-MS) shines light on the dissolution behaviour of several noble metals. We found that the electrochemical dissolution of Pt [2] and Rh [3] is predominantly a transient process. This finding is of critical importance particularly for storage of wind or sun energy via electrolysis, since these are intermittent processes. The strong fluctuations in power output will unavoidably lead to significant number of start/stop cycles and enhance dissolution. On the contrary Au behaves quite differently, as it intensively dissolves at constant potential or anodic currents [4]. Currently, dissolution of Ir, Ru, Rh, Pt, Pd, and Au, all noble metals considered for acid electrolysis, is also studied. To merge fundamental and applied aspects of noble metals oxidation and corrosion the insight is made on smart experimental planning. Aiming to simulate conditions of stationary electrolysis, non-stationary electrolysis as well as start-up and shut-down of an electrolytic cell, the experiments are conducted under constant potential, constant current and potential cycling. The eventual goal of the project is better understanding of noble metal dissolution which should result in design both active and stable oxygen electrocatalysts.