Coupling of a Scanning Flow Cell to an Online Electrochemical Mass Spectrometer
The time and cost efficient analysis of electrochemical catalysts is of major importance for the development of energy efficient clean technologies like fuel cells, electrolyzers or the electrochemical CO2 reduction. Characterization and valuation of electrochemical catalysts is only possible when three main qualities are taken into account (1) the activity, which indicates how efficient a catalyst is, (2) the stability, which reveals durability of a catalyst under certain operation conditions and (3) the selectivity, which relates to the product distribution at certain potentials or currents. The last point is especially important for reactions like the electrochemical CO2 reduction, where many different products are formed at similar potentials.
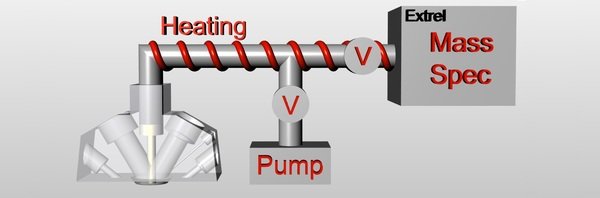
After the development of an electrochemical Scanning Flow Cell (SFC) for activity measurements, which is able to automatically screen applied potential/current, electrolyte, temperature and catalysts, and its coupling to an inductively coupled plasma mass spectrometer (ICP-MS) for stability measurements through quantitative dissolution analysis, we now coupled the SFC to an online electrochemical mass spectrometer (OLEMS). The new setup is able to determine the potential/current dependent selectivity of catalysts.
The original SFC design was changed to fit in a tip (white part in picture) from the top of the cell and install a PTFE membrane (20 nm pore size) at the bottom of it. The tip is directly connected to a mass spectrometer (Max300 LG, Extrel CMS, Pittsburgh, USA) and volatile products evolving on the catalyst are evaporated into the vacuum system and are analysed time resolved respectively potential/current dependent. When the cell is approached to the working electrode, the membrane is positioned only 50 to 100 µm away from the surface. This results in high product concentration and fast response times concerning the mass spectrometer signal. The counter electrode is made out of a carbon pipe and placed at the outlet of the cell to avoid influence of products evolved by the counter reaction on the mass spectrometer response. The reference electrode is mounted at the inlet of the SFC and the connection channel is positioned close to the surface to reduce the ohmic drop. Due to the extended channel diameter of 3mm the cell can also handle reactions, where heavy gas evolution would normally block the current flow.
While this setup was first of all built and is successfully used for the electrochemical CO2 reduction, it now has a much broader application range, not least because of the general ability of the SFC to use different kind of catalysts, like bulk poly or single crystals, thin films and nanoparticles. Starting from analysis of hydrogen and oxygen evolution reaction on noble metals or during classical corrosion over carbon corrosion to isotope labelled measurements, this setup makes a great contribution to our groups research and will hopefully also help to accelerate development of eco-friendly technologies, to ensure CO2 neutral energy supply.
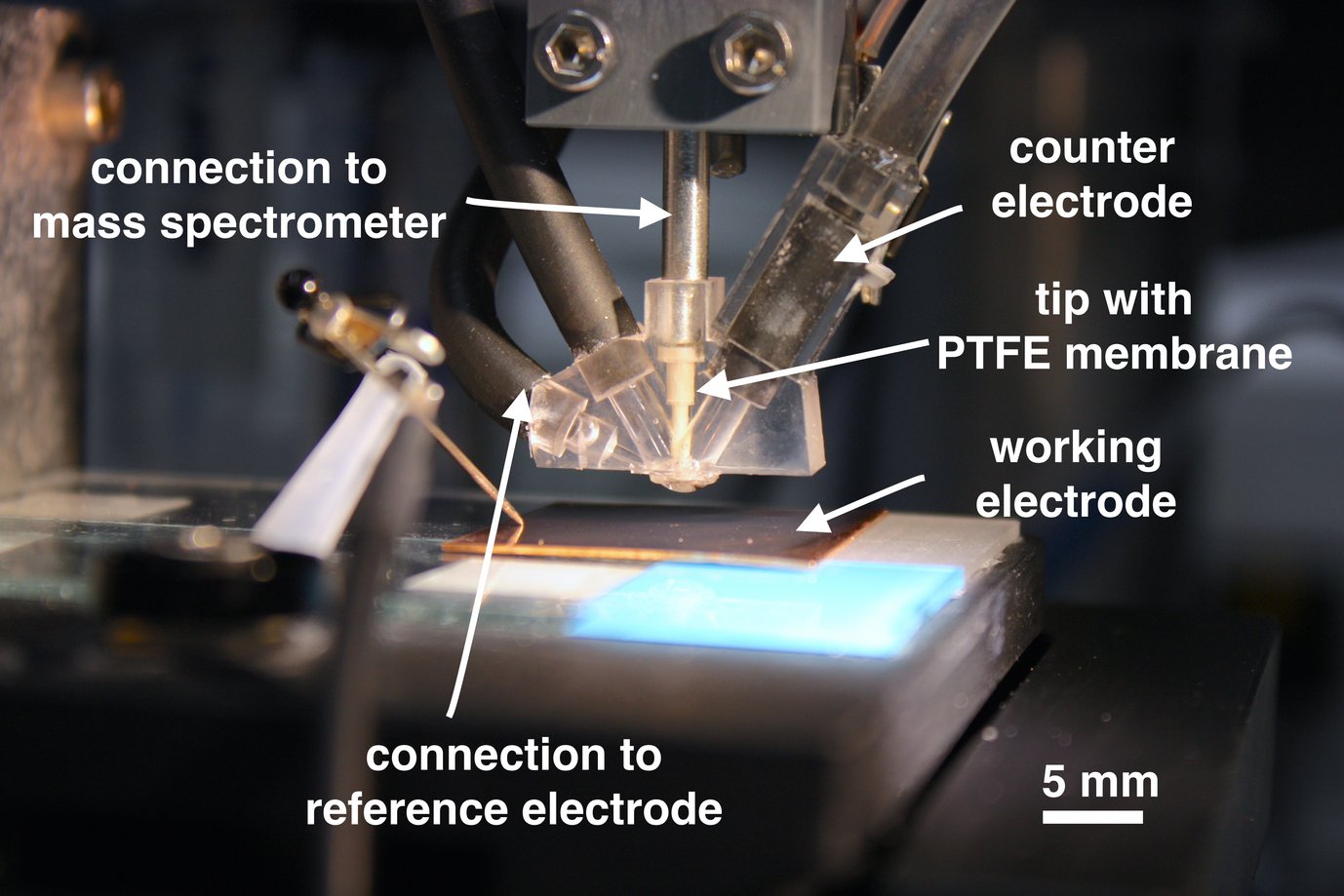
Coupling of an electrochemical scanning flow cell (SFC) to an online electrochemical mass spectrometer (OLEMS). A tip (white part) is introduced from the top of the cell and a PTFE membrane (20 nm pore size) is placed at its bottom. When the cell is approached to the working electrode, the distance between membrane and electrode is only 50-100 µm. Volatile products evolving on the electrode evaporate through the membrane and are analysed time respectively potential/current resolved by the mass spectrometer.
