Oxygen Evolution Reaction
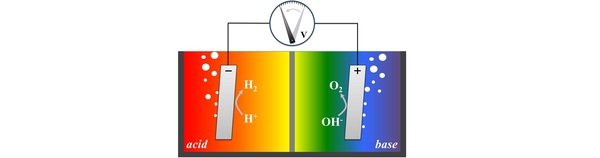
Water electrolysis as a key technology for the storing of renewable electricity is one of the principal research areas in our group. Targeted efficiency of water electrolysis is still serious challenge predominantly due to the limitations in the performance of anode reaction – oxygen evolution reaction. Namely, oxygen evolution reaction exhibits unusually low rate constants and at the same time induces structural changes into electrode materials that lead to drop in performance and ultimately electrode breakdown. Consequently, to comprehend complexity and to improve performance of this essential reaction we conduct research in several important sub-areas:
a) fundamental studies on metal/water interactions and oxide/water interactions including following metals and their oxides: platinum, gold, ruthenium, iridium, rhodium and palladium.
b) introducing new activity and stability descriptors for various types of oxides (rutiles, spinels, perovskites…)
c) synthesis and characterization of mixed binary and ternary oxides (RuOx and IrOx based with valve metal-oxide supports)
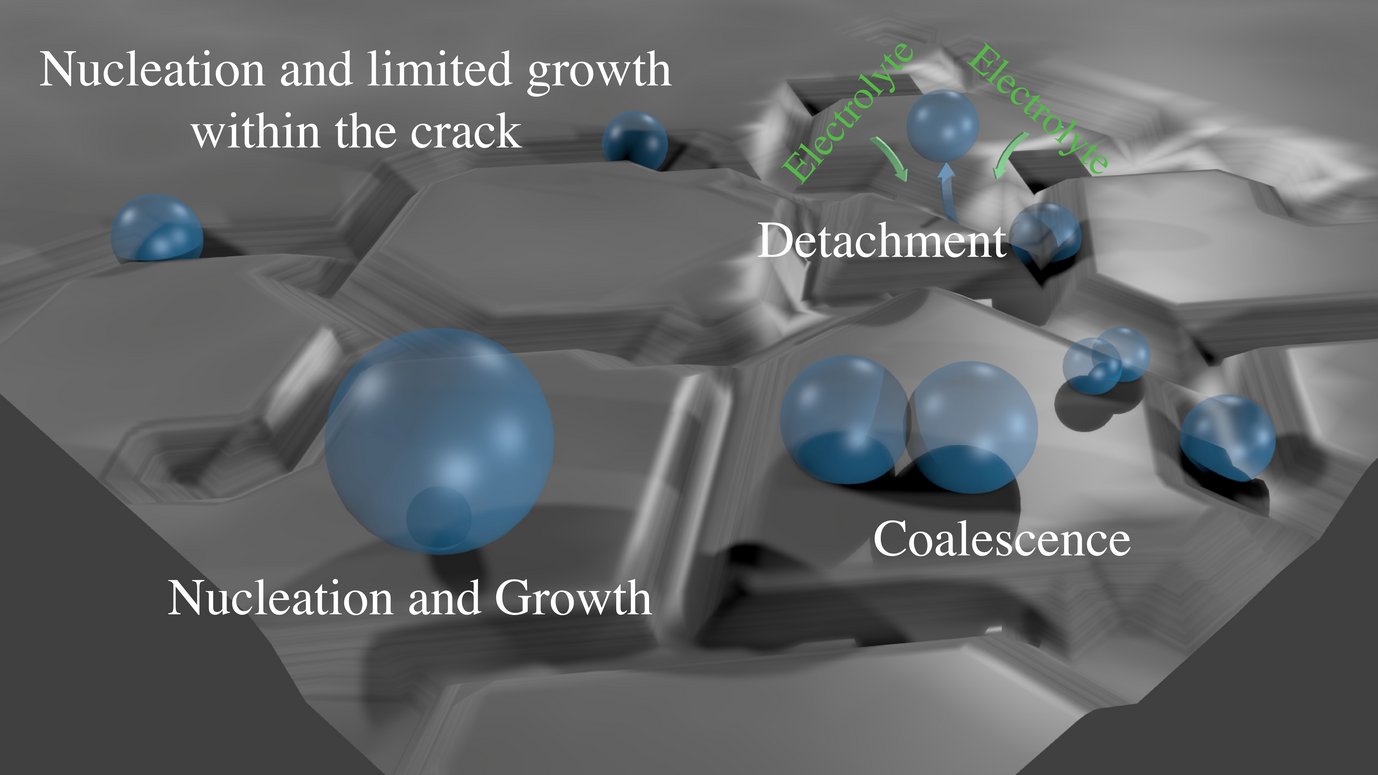
d) studies on gas-bubble effect and mass transport
e) development of principles for rational design of electrode morphology on micro- and nanoscale
f) investigation of activity-stability interrelations for nonnoble materials (Mn-based oxides, Ni-based oxides etc…)
g) from future perspective, substantial efforts will be dedicated to investigation of self-recovering catalysts and studies of reaction mechanism using isotope labeling.