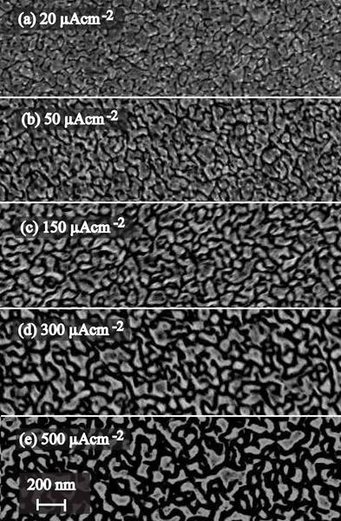
Electrochemical texturing of Al-doped ZnO
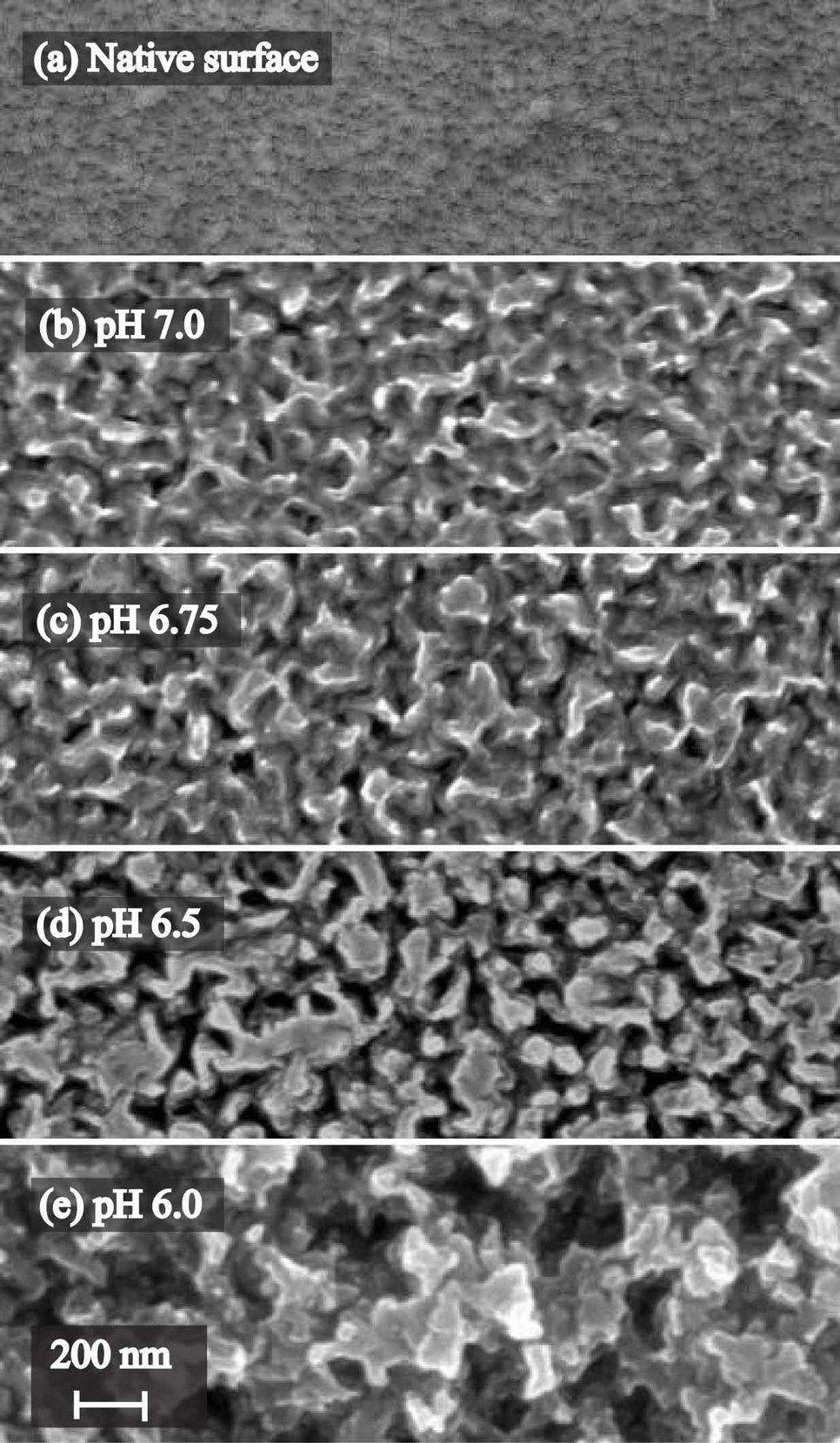
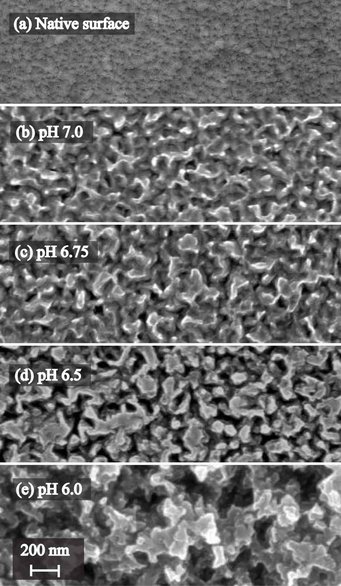
Transparent conducting oxides (TCO) play an important technical role, for instance as an electrical contact in thin film solar cells. ZnO was found to be a promising material for photovoltaics due to its optical end electrical characteristics, with the material optimization process presently ongoing. In case of thin film solar cells, a simple transmission of light is not desired, but rather a scattering which leads to a prolongation of the light path through the absorber layer (e.g. silicon) [1]. This tuning of the optical properties by surface texturing is usually achieved by a chemical etching step. The image beside shows the surface morphology as a function of the solution pH in acetate buffer (0.1 M) for an etch time of 500 s. As evident from the scanning electron images, a significant roughening of the surface occurs, with the etch pits most probably originating from the grain boundaries.
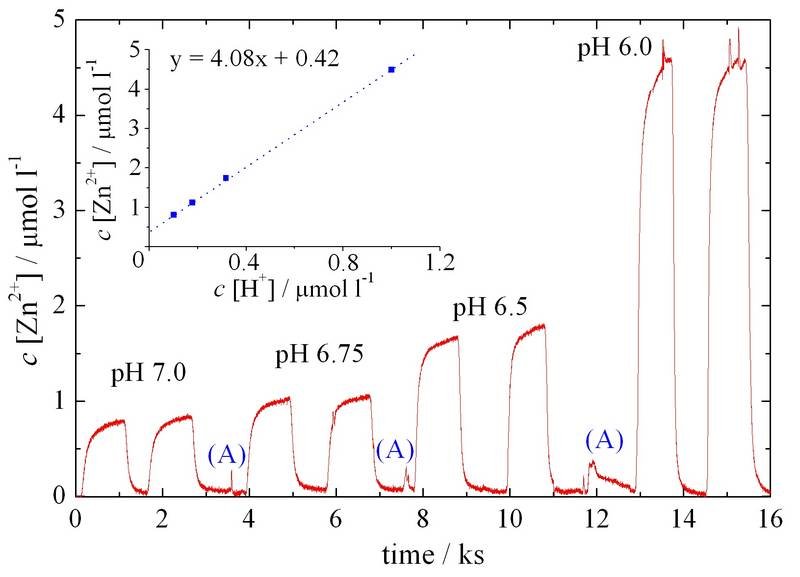
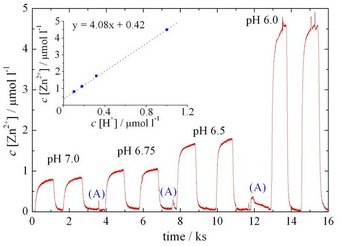
As shown by downstream analysis of zinc ions using a SFC setup [2] (see figure below), a linear relation between etch rate and proton concentration was observed. The reaction mechanism was furthermore shown to be diffusion limited by proton transport, both sensible to the solution pH and buffer concentration.