Ab initio study of corrosion: Adsorbate phases on surfaces and phase diagrams
The project aims to study corrosion, a detrimental process with an enormous impact on global economy, by combining denstiy-functional theory calculations with thermodynamic concepts.

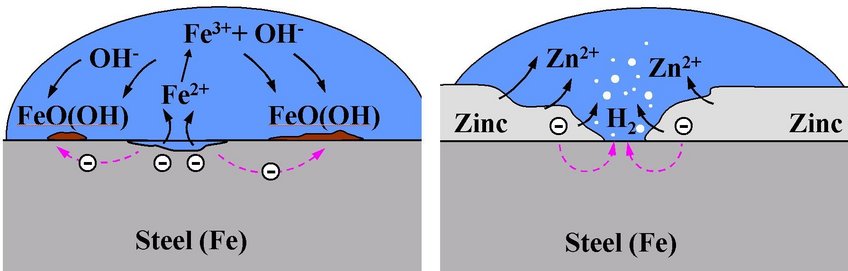
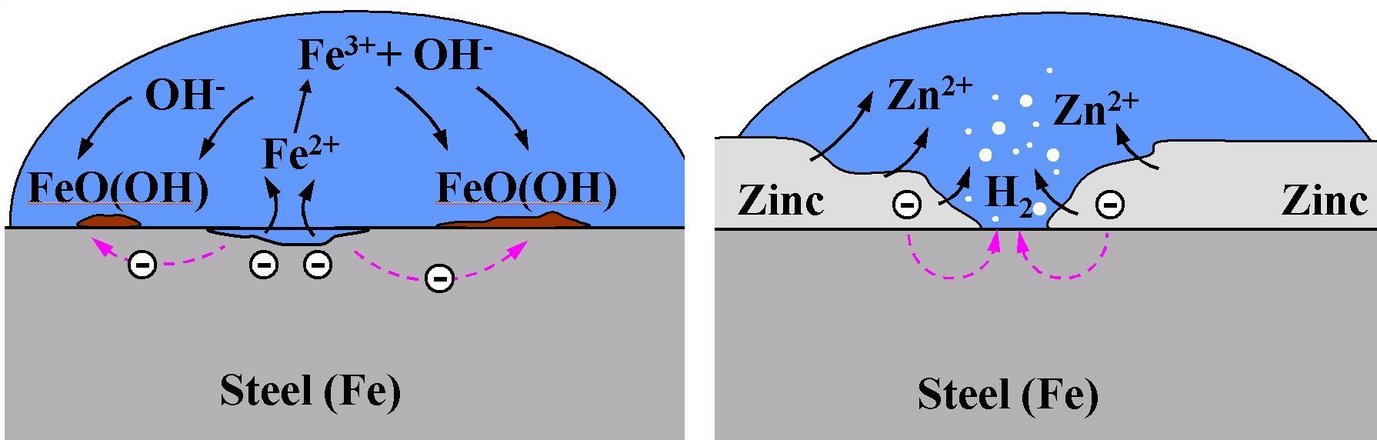
Corrosion is a technologically important, albeit unwanted process, which has an enormous impact on global economy. Corrosion leads to deterioration of essential material's properties. It is a chemical reaction of the material with its environment as a result of which electric current flows. Two reactions take place: an anodic reaction, also called oxidation process (the term used in this sense is not concerned with a reaction involving oxygen), which produces electrons and the electron consuming reaction, which is termed cathodic. Corrosion can take place in as different media as atmosphere, solution or soil. The formed corrosion product (corroded form of the material) represents then the thermodynamic ground state for the given environmental conditions.
Metals, which are not the thermodynamically stable phase within an oxidising environment, are particularly susceptible to corrosion. When a metal comes into contact with e.g. water a corrosion cell builds-up. In it, the liquid acts as an ion-conductor, while the metal is responsible for the transport of electrons from a local anode, at which metal ions are oxidised and go into solution, to a local cathode, at which ions are reduced and electrons consumed. As long as the flux of metal ions going into solution exceeds the flux of metal ions being reduced at the surface, the material corrodes.
A material can be protected against corrosion, as achieved, e.g., by galvanisation of steel, in which case a zinc layer is applied on the steel surface. This layer offers threefold protection:
(i) it constitutes a protective layer, which shields the steel surface from the environment,
(ii) due to the lower corrosion potential of zinc the zinc layer is the first to corrode following a breakthrough,
(iii) the Zn-corrosion products precipitating on the steel surface inhibit oxygen reduction significantly, thus relieving the corrosion pressure from the zinc coating.
Understanding the processes and mechanisms involved in the corrosion process is essential for the development of effective corrosion protection concepts and vital to the rational design of efficient, long life materials. Improving corrosion protection is, however, often a question of trial and error approaches, largely based on empirical observation rather than sound theoretical understanding. An accurate characterisation of the solid/liquid interface and the occuring elementary processes would constitute an essential step towards gaining in-depth insight into the functionality of a corrosion cell.
In this project we aim to study the corrosion process by explicitly considering the electrolyte/oxide interface.However, the prominent role which water plays in a corroding system makes the theoretical description rather challenging. We have developed an approach, derived from the defect-chemistry in semiconductors, which enables us to derive factors governing corrosion, such as the electrode and the pH-Scale, based on density-functional theory calculations.