Stainless steel for the hydrogen economy
Passivation strategy overcomes corrosion and hydrogen embrittlement
At a glance:
- Challenge: Up to now, stainless steel is prone to corrosion and embrittlement when used for hydrogen transport and storage
- Approach: Atomic-scale grain boundary passivation using nitrogen
- Result: 3.8× higher corrosion resistance and a 1.35× higher resistance to hydrogen embrittlement than 316L steel
- Impact: Enables safer, cost-efficient hydrogen storage and transport with established manufacturing routes

Hydrogen is a cornerstone of future climate-neutral energy systems. Yet storing and transporting hydrogen safely remains a major materials challenge. Stainless steels are attractive candidates because they are strong, affordable and widely used. Even advanced grades, however, are vulnerable to corrosion and hydrogen embrittlement, a process in which hydrogen penetrates the metal, weakens internal bonds and can ultimately lead to sudden failure. In a new study, an international research team led by the University of Science and Technology Beijing and the Max Planck Institute for Sustainable Materials (MPI-SusMat) has developed a novel austenitic stainless steel that addresses both challenges at once. The findings have now been published in the journal Science Advances.
Using nitrogen to protect grain boundaries
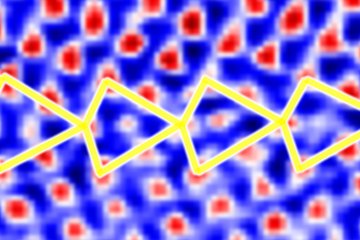
Grain boundaries are among the most vulnerable defects in metals.
They act as fast diffusion pathways for hydrogen and as active sites for electrochemical corrosion reactions. Hydrogen embrittlement occurs when mobile hydrogen accumulates at these interfaces, creating local stress concentrations that can trigger decohesion and cracking. Corrosion, meanwhile, results from electrochemical interactions between the material’s microstructure and its surrounding environment.
“The challenge was to develop a stainless steel that remains mechanically reliable in the presence of hydrogen while also offering high corrosion resistance,” explains Dierk Raabe, director at MPI-SusMat and, corresponding author of the study. “At the same time, the material had to be cost-efficient and compatible with established manufacturing routes. Because grain boundaries are the most vulnerable defects, we focused on preventing hydrogen ingress at precisely these locations.”
Atomic-scale passivation delivers long-term protection
Rather than relying solely on a conventional surface oxide film, the researchers introduced nitrogen atoms directly into the grain boundaries of the steel. This atomic-scale decoration effectively blocks hydrogen access and suppresses defect activity before damage can occur.
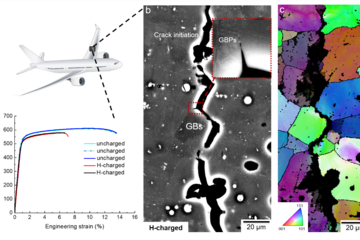
The result is an alloy (Fe-20Cr-9Ni-2.5Mn-1.6Mo-1Cu-0.2N) that shows a 3.8-fold increase in corrosion resistance and a 1.35-fold improvement in resistance to hydrogen embrittlement compared with commercial 316L stainless steel.
A scalable and sustainable solution
Unlike strategies that trap hydrogen in precipitates, which can quickly become saturated, grain boundary passivation offers long-term protection.
The new alloy is cost-effective, compatible with established industrial processing routes, and comes with a lower carbon footprint than many high-performance alternatives. By combining durability, hydrogen tolerance and affordability, it provides a realistic pathway towards safer pipelines, tanks and components for hydrogen transport and storage.
This atomic-scale design strategy could be extended to other alloys, opening new opportunities for durable materials across energy, chemical and infrastructure applications.
Original publication
Author: Yasmin Ahmed Salem