Decomposition of the single-phase high-entropy alloy CrMnFeCoNi after prolonged anneals
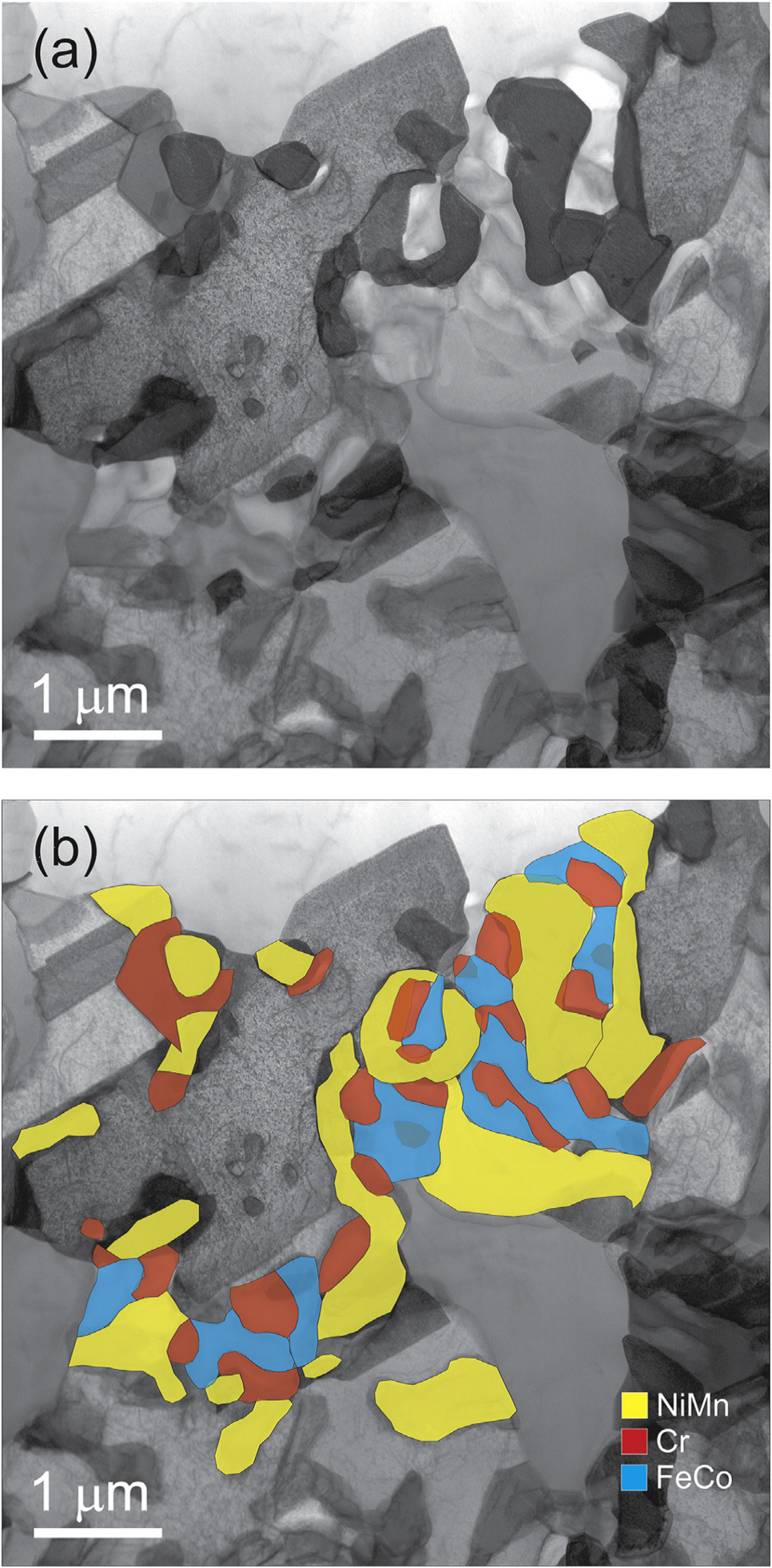
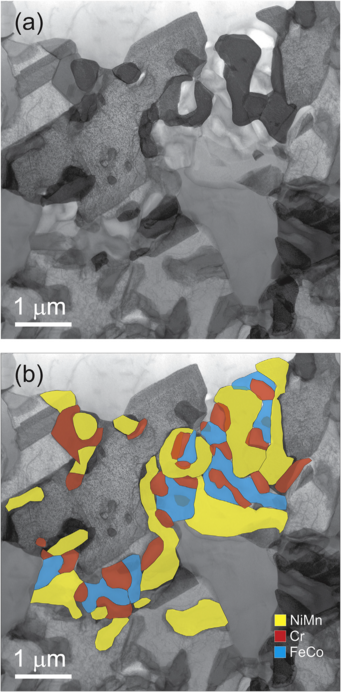
STEM and EDX analysis of a FIB lamella extracted from the CrMnFeCoNi alloy after a 500°C anneal for 500 days. (a) STEM BF contrast reveals the presence of several secondary precipitates on a grain boundary of the CrMnFeCoNi matrix. (b) EDX maps superimposed on the image in (a) show that the precipitates have three distinct chemical compositions.
The equiatomic HEA, CrMnFeCoNi, is a face-centered cubic (FCC) prototype of this class and has attracted much attention recently because of its good mechanical properties. In this collaborative project conducted together with the group of Prof. Easo George we evaluate its phase stability after very long anneals of 500 days at 500-900°C during which it is reasonable to expect thermodynamic equilibrium to have been established. Microstructural analyses were performed using complementary analysis techniques including scanning and transmission electron microscopy (SEM/TEM/STEM), energy dispersive X-ray (EDX) spectroscopy, selected area electron diffraction (SAD), and atom probe tomography (APT). We show that the alloy is a single-phase solid solution after homogenization for 2 days at 1200°C and remains in this state after a subsequent anneal at 900°C for 500 days. However, it is unstable
and forms second-phase precipitates at 700 and 500°C. A Cr-rich s phase forms at 700°C, whereas three different phases (L10-NiMn, B2-FeCo and a Cr-rich body-centered cubic, BCC, phase) precipitate at 500°C. These precipitates are located mostly at grain boundaries, but also form at intragranular inclusions/pores, indicative of heterogeneous nucleation. Since there is limited entropic stabilization of the solid solution state even in the extensively investigated CrMnFeCoNi alloy, the stability of other HEAs currently thought to be solid solutions should be carefully evaluated, especially if they are being considered for applications in vulnerable temperature ranges.