Understanding the variable composition in iron oxides
Iron oxides and hydroxides of variable stoichiometry occur at the essential stages of iron ore reduction and ferrous metal corrosion (rusting). We aim at building up a fundamental understanding of how the ions (Fe2+, Fe3+, O2-, OH-) may arrange and rearrange to achieve such a broad composition range, in order to optimize the conditions to improve reduction processes and prevent corrosion.
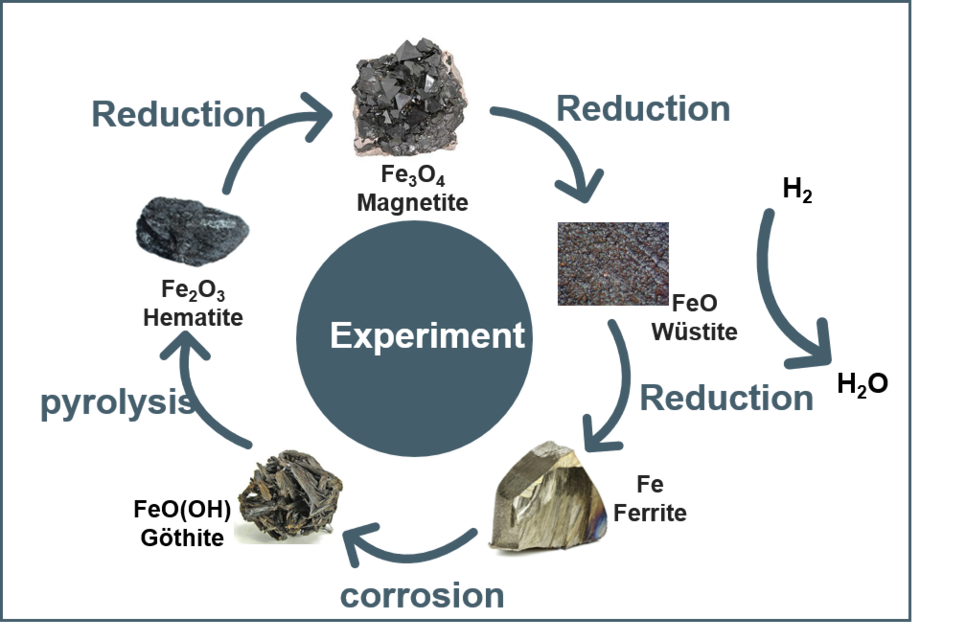
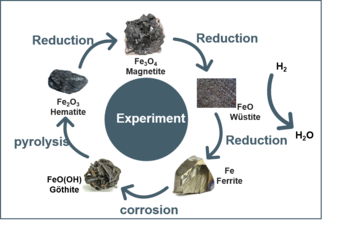
With increasing awareness of global warming issues and sustainability, researchers have raise the interest in iron ore reduction in a sustainable way using clean energy source i.e. hydrogen (H₂) to avoid CO2 emissions. The typical transformation pathway of iron ore reduction follows the sequence: Hematite (Fe2O3) → Magnetite (Fe3O4) → Wüstite (FeO) → Ferrite (α-Fe) + H2O. It involves not only formula changes (FexO, x = 0.67–1) but also crystal structure transformations (phase transitions), non-stoichiometric defects (point defects, defect clusters), charge transfer (Fe3+ → Fe2+ → Fe), and cation diffusion (Fe3+/Fe2+). These complex phenomena drive us to explore the underlying physical principles.
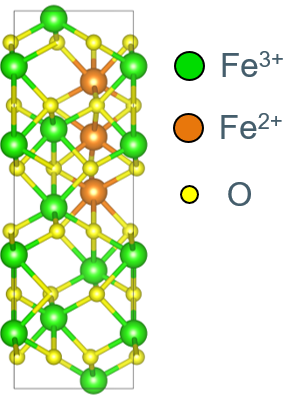
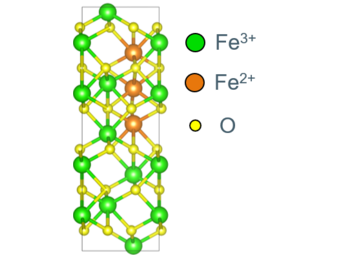
We currently focus on the properties of intrinsic point defects such as interstitials and vacancies as the building blocks for off-stoichiometry. In the ionic picture, the oxygen atoms appear as O2- ions, and the charges are compensated by Fe2+ and Fe3+. While the positions of the Fe atoms is pretty clear - they prefer octahedral (6-fold coordinated) environments, but can also appear in tetrahedral (4-fold) sites, the change in charge state is part of the electronic structure. The Fe3+ ions have 5 d-electrons of the same spin. Fe2+ has one more d-electron of opposite spin. Standard density functional theory fails to describe the localization of electrons near single Fe atoms, treating the excess spin-down electrons as delocalized metallic electrons in conflict with experimental results. We employ Hubbard corrections (DFT+U) to ensure a correct electronic structure with localized electrons. Yet, we also aim to control the place of electron localization to be able to study different Fe2+/Fe3+ configurations systematically.
Follow links to see more projects from the Defect Chemistry and Spectroscopy and from the Electrochemistry and corrosion groups, respectively.