Water splitting catalysts at the atomic scale
In this project we work on the corelative characterization of the atomic structure and composition of water-splitting catalysts. We aim to better understand reaction and degradation mechanisms of Ir-based catalysts for the oxygen evolution reaction (OER) by establishing structure-function relationships at the atomic scale.
The world’s transition towards a more sustainable energy landscape strongly relies on the development of efficient energy conversion systems, such as water electrolyzers. The commercial upscaling of such devices is widely hindered by the sluggish kinetics of the OER and low stability of most catalysts under the harsh electrochemical OER conditions. Iridium oxides are one of a few materials that exhibit a reasonable stability-activity trade off, however, the low abundance and consequential high price of Ir requires its optimal utilization in such devices. Hence, the fundamental knowledge of degradation processes is crucial for the optimization of the long-term stability of OER catalysts.
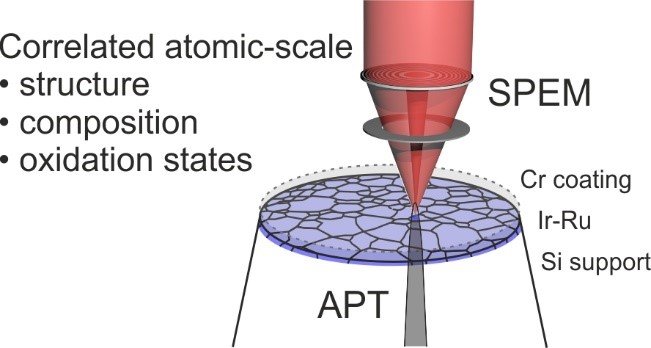
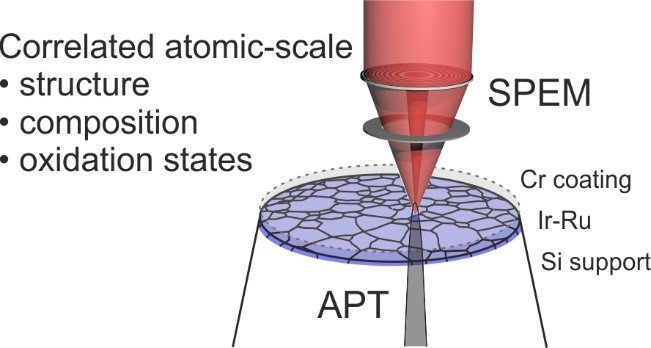
At the core of this project is the investigation of catalysts using atom probe tomography (APT) that enables the characterization of the (near-) surface region of a material at the atomic scale, in combination with electrochemical measurements which yield insights into activity-stability relationships of the material. We use isotope-labelling techniques to trace e.g. the exchange of atoms between the material and the electrolyte or the degree of hydroxylation during the reaction. Further, we employ surface spectroscopy techniques such as X-ray photoemission spectroscopy (XPS) or scanning photoemission electron microscopy (SPEM) to investigate the electronic structure of the surfaces in question (Fig. 1). The suite of analytical techniques is complemented with further scanning electron microscope techniques including electron backscatter diffraction and energy-dispersive X-ray diffraction, as well as transmission electron microscopy.
Model systems are employed and developed to rationalize observations made on real systems. This approach allows us to systematically investigate the influence of e.g. crystalline defects or surface orientation on the distribution of solute elements, the local electronic structure, reactivity or degradation. In summary, we aim to find suitable atomic scale descriptors for the observed electrochemical properties and related degradation phenomena of the investigated materials.