Designing Ultrahigh Strength Steels with Good Ductility by Combining Transformation Induced Plasticity and Martensite Aging
Steels with a high ultimate tensile strength (UTS) above 1 GPa and good ductility (total elongation (TE) of 15-20% in a tensile test) are of greatest relevance for lightweight engineering design strategies and corresponding CO2 savings. In this project we work on a novel design approach for precipitation hardened ductile high strength martensitic and austenitic-martensitic steels (up to 1.5 GPa strength).
Dierk Raabe, Dirk Ponge, Olga Dmitrieva, Benedikt Sander
Abstract
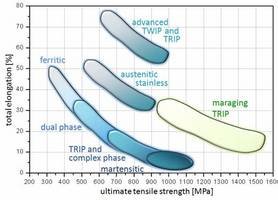
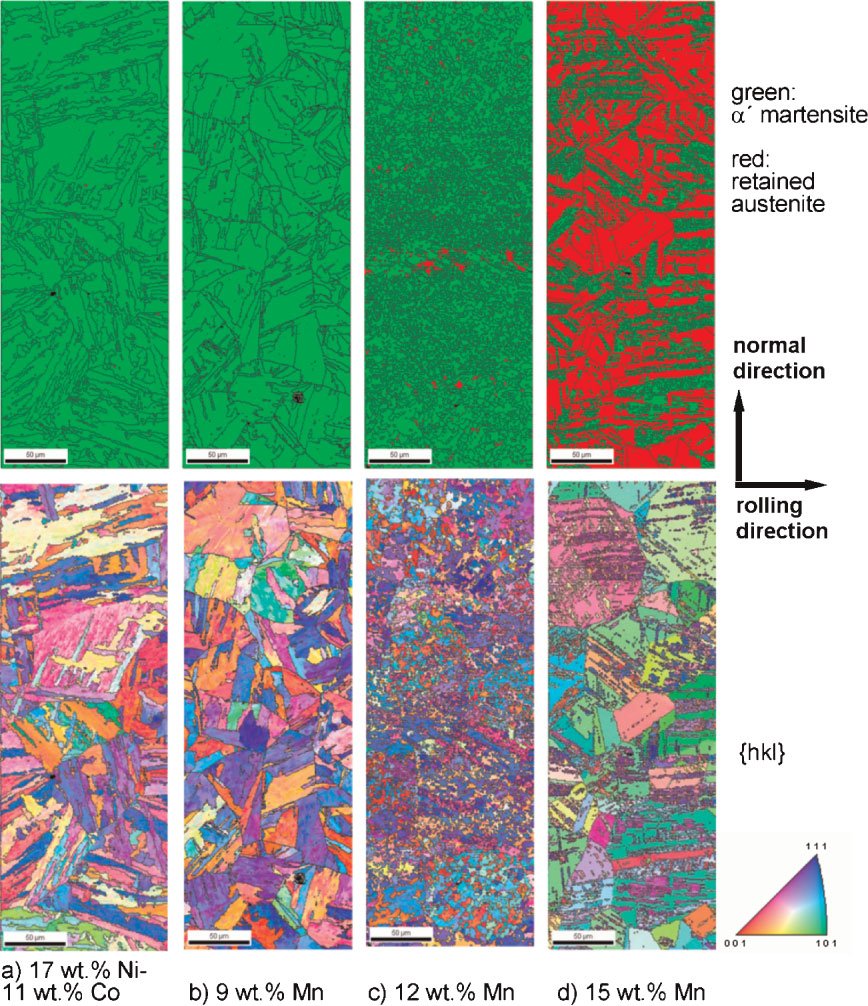
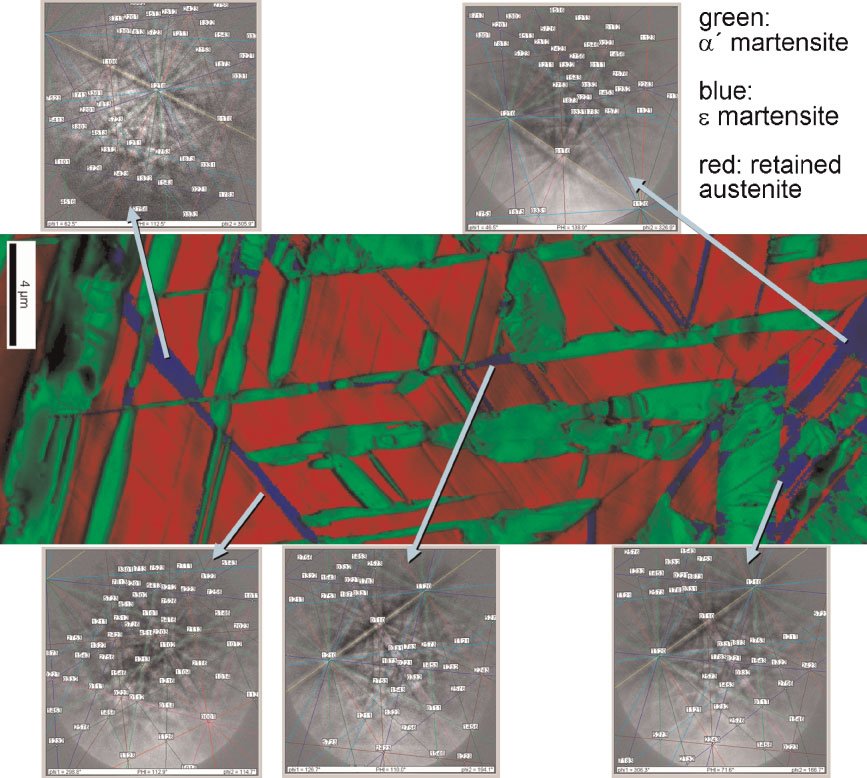
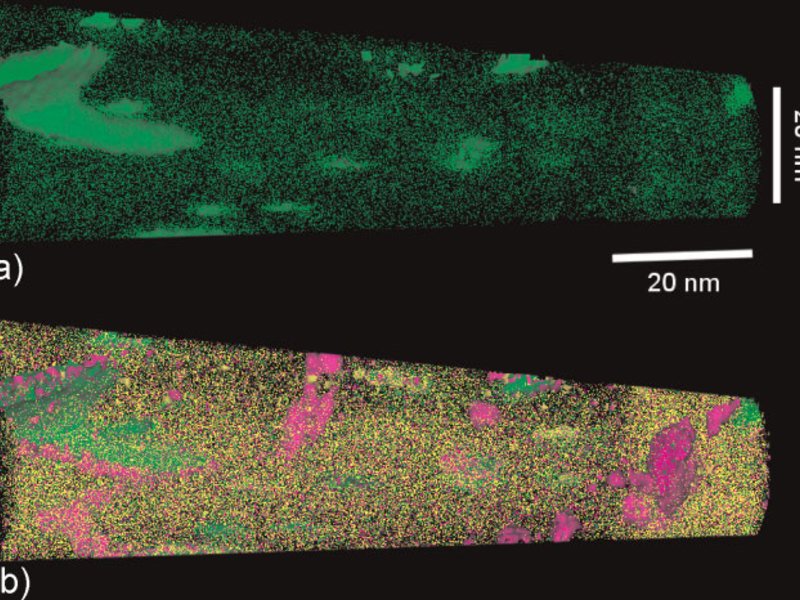
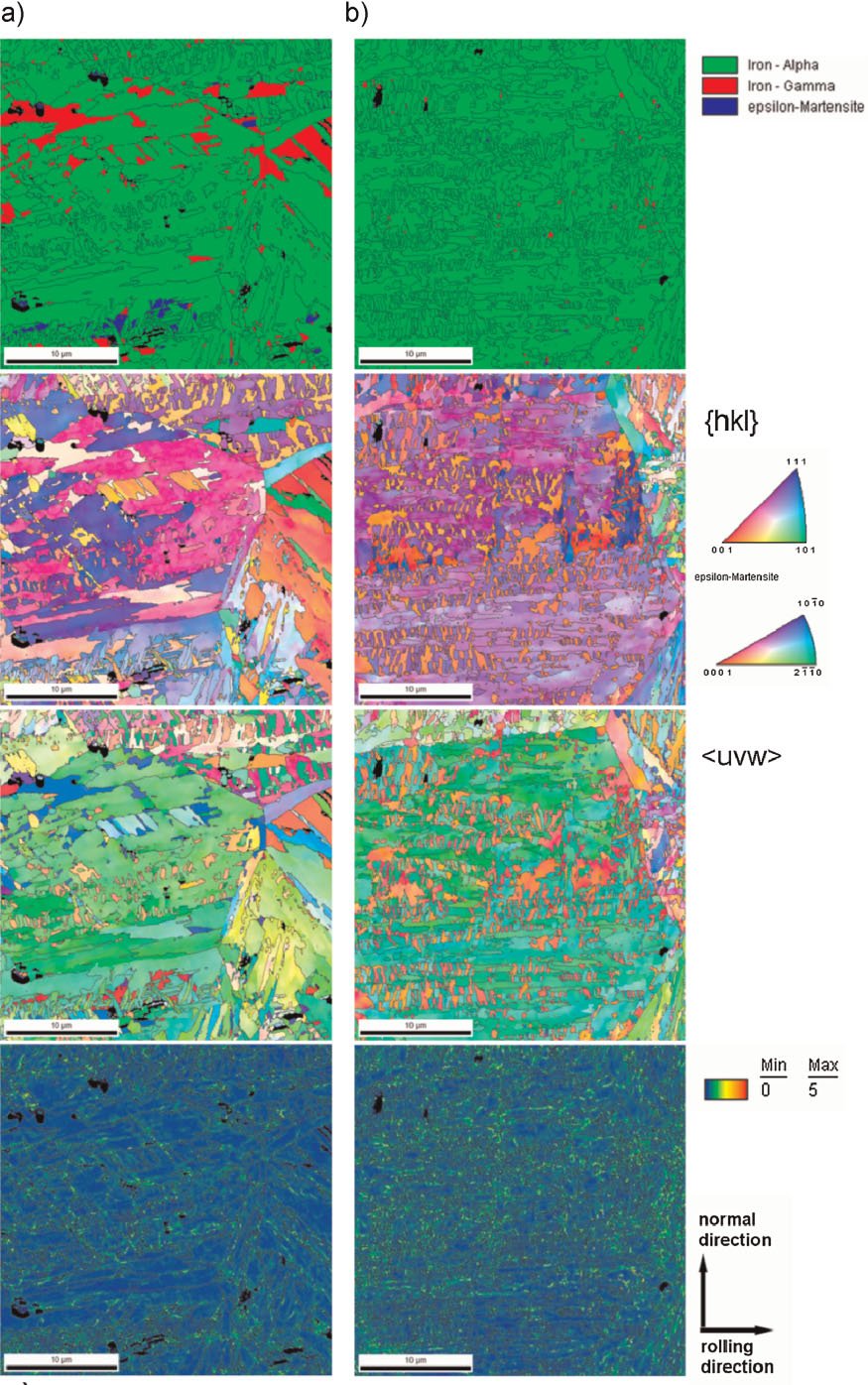
Figure 1: Overview of the typical strength-ductility profiles of different types of steels. The strength is expressed in terms of the ultimate tensile strength measured during tensile testing and the ductility is expressed in terms of the total sample elongation. The data represent regimes such as published in the references given below. TRIP: transformation-induced plasticity; TWIP: twinning-induced plasticity; Complex phase: multiphase steels (e.g. austenitic-martensitic steels which may contain also bainite); maraging TRIP: new steel concept that includes hardening mechanisms based on transformation induced plasticity and the formation of intermetallic nanoparticles in the martensite during aging. The approach leads to an unexpected simultaneous increase in both strength and total elongation (green area) enhancing the regime of formable ultrahigh strength steels by 0.5 GPa.