Mechanisms of self and impurity diffusion in Fe-Al intermetallic compounds
The diffusion mechanisms in ordered binary alloys are more complicated than in materials with only one atom species. Several mechanisms, including, e.g., triple defect jump cycles, have been suggested in the literature. Within this project, we resolve which of them is energetically most favorable in FeAl and use the calculated barriers for large scale simulations.
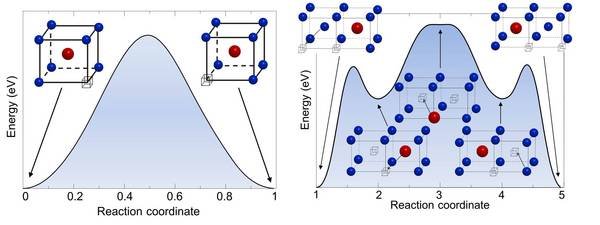
Schematic representation of the minimal energy path for two jump processes in FeAl: (left) the next-nearest neighbor jump of a Fe atom into a vacancy and (right) the triple defect mechanism. The Fe atoms are marked as blue balls, Al atoms red balls and the vacancy is symbolized by the box. For the second mechanism intermediate (meta-)stable structures are marked by the arrows.
© Niko Sandschneider, Max-Planck-Institut für Eisenforschung GmbH
