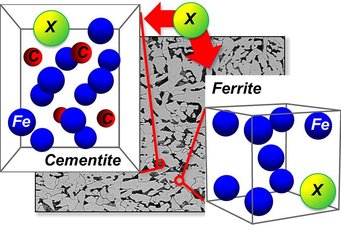
Influence of alloying elements on the thermodynamic stability of cementite in ferritic steels
The thermodynamic stability of cementite can be controlled with chemical alloying: for instance, Si and Al are commonly used to suppress cementite formation, whereas Cr enhances it. In this project we employ a combination of parameter-free first-principles calculations and concepts of equilibrium thermodynamics to get insights into physical mechanisms which influence the thermodynamic stability of cementite upon alloying.
Introduction
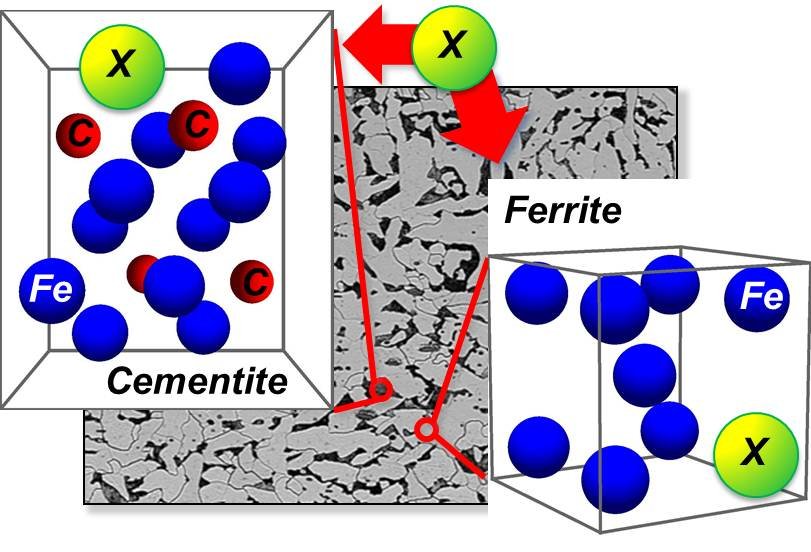
Knowledge-based control of the steel’s microstructure is critical for successfully designing new steels showing improved toughness, strength and ductility. In carbon steels, the microstructure is essentially defined by the distribution of defects, impurities and carbides. Among the latter cementite, as the predominant type of carbide precipitates, plays a key role in influencing mechanical and physical properties in carbon steels. Thus, suppression or enhancement of cemenite formation and its morphology during the steel fabrication is one of the important mechanisms which allow one to tailor the mechanical properties of steels. Importantly, since cementite is only a part of the complex microstructured alloy, controlling cementite precipitation does not only mean to control the amount and distribution of cementite in steel, but also the formation of other phases and is, therefore, important for many steel treatments such as quenching and partitioning [1].
Motivation
The thermodynamic stability of cementite can be controlled with chemical alloying: for instance, Si and Al are commonly used to suppress cementite formation, whereas Cr enhances it [2,3]. Despite of various theoretical and experimental studies, the unambiguous detailed understanding of the mechanisms which (de)stabilize cementite in presence of alloying elements, in particular considering temperature-driven effects, is in many cases missing.
Aim
In this project we employ a combination of parameter-free first-principles calculations and concepts of equilibrium thermodynamics to get insights into physical mechanisms which influence the thermodynamic stability of cementite upon alloying. In contrast to most of the previous theoretical models, we assume that cementite is a part of the thermally equilibrated cementite-ferrite system, and not an isolated phase. Our model accounts for temperature-driven effects taking the configurational part of the entropy into account, and relies on the first-principles calculations for model parameters. On the later stages of the project further types of the temperature-driven excitations (like, e.g., phonons and magnons [4]), as well as kinetic effects, will be taken into account.
References
[1] F.C. Rizzo, K. Matlock J.G. Speer, D.V. Edmonds: Current Opinion in Solid State and Mat. Sci. 8 (2004) 219-237.
[2] D.V. Edmonds, H.K. Bhadeshia: Met. Sci. 17 (1983) 420–425.
[3] E.C. Bain: The Alloying Elements in Steel. American Society of Materials (1939).
[4] B. Hallstedt, D. Djurovica, J. von Appen, R. Dronskowski, A. Dick, F. Körmann, T. Hickel, J. Neugebauer, Calphad 34 (2010), 129.