Ab initio based finite-temperature thermodynamics of alloys
Phase stabilities of multicomponent material systems are best simulated with CALPHAD. The underlying databases are mainly obtained from calorimetric measurements, but suffer often from the fact that for certain phases an experimental approach is not feasible or not conclusive. Here, ab initio calculations emerge as an alternative, which is carefully investigated for the Al-Mg-Si-Cu and Al-Sc system within this project.
Motivation
Al-Mg-Si-Cu and Al-Sc alloys are widely used in engineering applications due to their excellent mechanical properties: low density, high hardness and melting temperature. To tailor these properties to specific needs, it is necessary to model and describe the thermodynamic properties of its unary and binary phases and subsequently extrapolate those properties to ternary and higher-order systems [1]. The quantitative simulation methods used to address this challenge (e.g. CALPHAD; CALculation of PHAse Diagrams) essentially rely on very precise thermodynamic potentials, which are in most cases derived from calorimetric experiments. In cases of non-existent experimental data, to determine the accurate position of metastable phase boundaries (the reliability of experiments is limited due to the transient nature of metastable phases [2]) or simply to verify experiments, ab initio calculations emerge as an alternative and have proven to be able to provide exact thermodynamic functions [3a].
Aims
The primary goal of this project is to provide highly accurate ab initio free energies as a function of temperature and molar volume for selected binary, ternary and quaternary phases of multicomponent Al alloys. A major challenge of this work is to take all entropic contributions as well as their coupling simultaneously into account and thus to write the free energy F(V,T) as:
Here Fvib is the free energy due to atomic lattice vibrations, Fel corresponds to the electronic contribution to the free energy, Fah to the explicitly anharmonic free energy, Fvac to the free energy due to vacancies and and Fel-vib is the electron-phonon coupling contribution [3b]. Having obtained the thermodynamic potential F(V,T), we can derive various quantities : Gibbs free energies of formation, enthalpies, entropies, heat capacities, thermal expansions, vacancy concentrations etc.
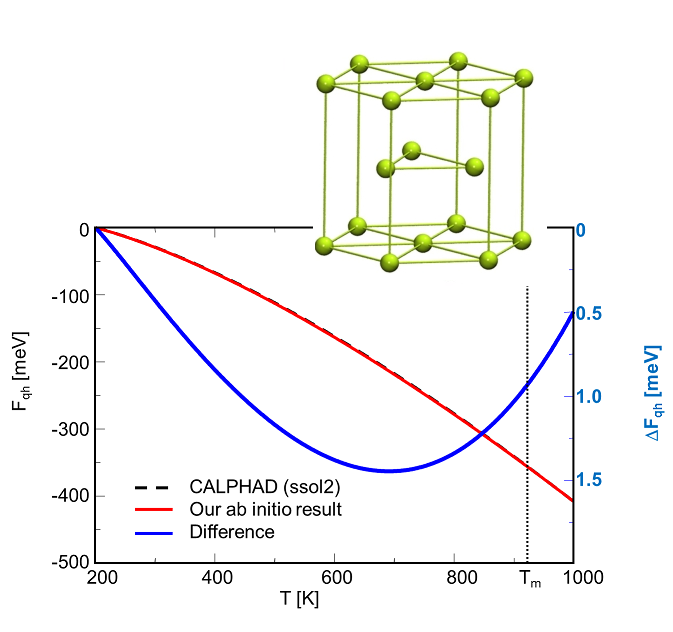
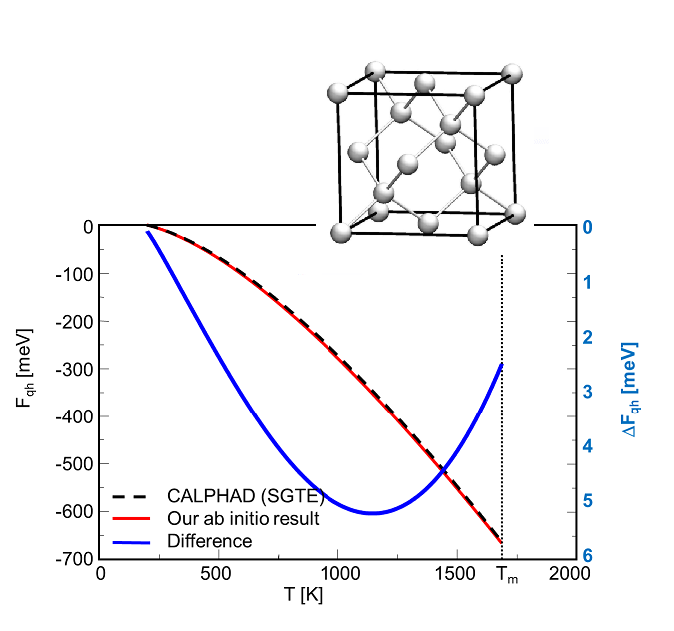
Application of ab initio based finite-temperature thermodynamic concepts to alloys
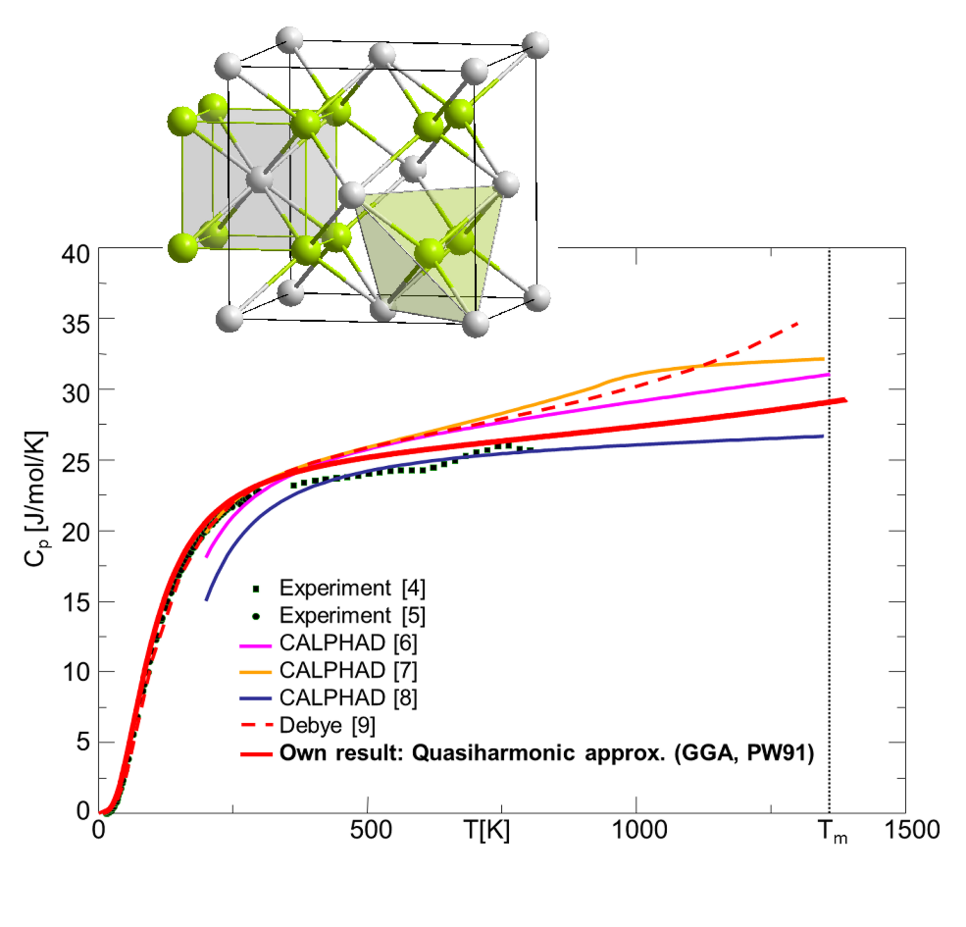
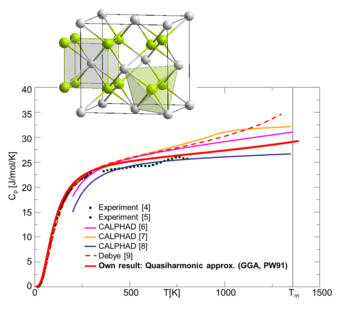
Comparison of ab initio computed heat capacity of Mg2Si with experiments and CALPHAD databases.
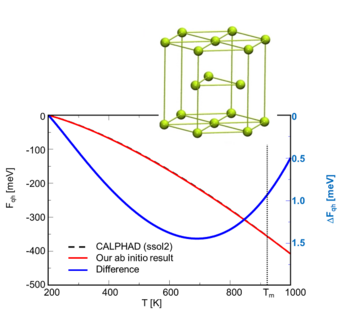
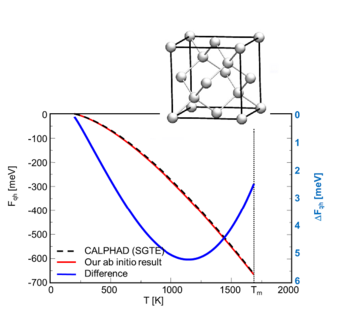
Example 1: Mg2Si --- Our methods have first been tested for the unary components, being also important for the determination of formation energies. Figs. 1a and 1b shows exemplary a comparison of the free energy of Mg(hcp) and Si(dc) calculated with ab initio and the CALPHAD method. We observe that the ab initio results are in excellent agreement with the CALPHAD evaluation.
We have, therefore, generalized our methods in a second step to the binary systems. The results for Mg2Si are shown in Fig. 2. For this material system, previous CALPHAD parametrizations (thin lines, colored) show large deviations for the heat capacity above room temperature, where only a limited amount of experimental data is available. The CALPHAD results are also not consistent with simulations based on the Debye model. A complete ab initio prediction of the thermodynamic property (thick red line inf Fig. 2) yields therefore valuable information about trustworthy trends at high temperatures.
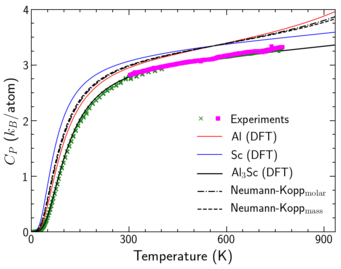
Example 2: Al3Sc --- In case of the interesting lightweight material system Al-Sc, we apply these concepts to study the heat capacity of Al3Sc. Due to their importance for precipitation hardening, the formation of these L12 ordered coherent Al3Sc particles has been extensively studied with DFT by us and with TEM and APT by our experimental partners. Their carefully performed calorimetric measurements allows us further to benchmark the accuracy of our predictions. Fig. 3 shows the ab initio predicted heat capacity that contains all finite-temperature contributions and yields an excellent agreement with the calorimetric measurements. One can clearly see that the empirical Neumann-Kopp predictions overestimate the results. In addition, to correctly describe the trends at temperatures close to melting, we also demonstrated the significance of electronic contributions and the electron-phonon coupling effects and the deviations from empirical approaches like the Debye model.
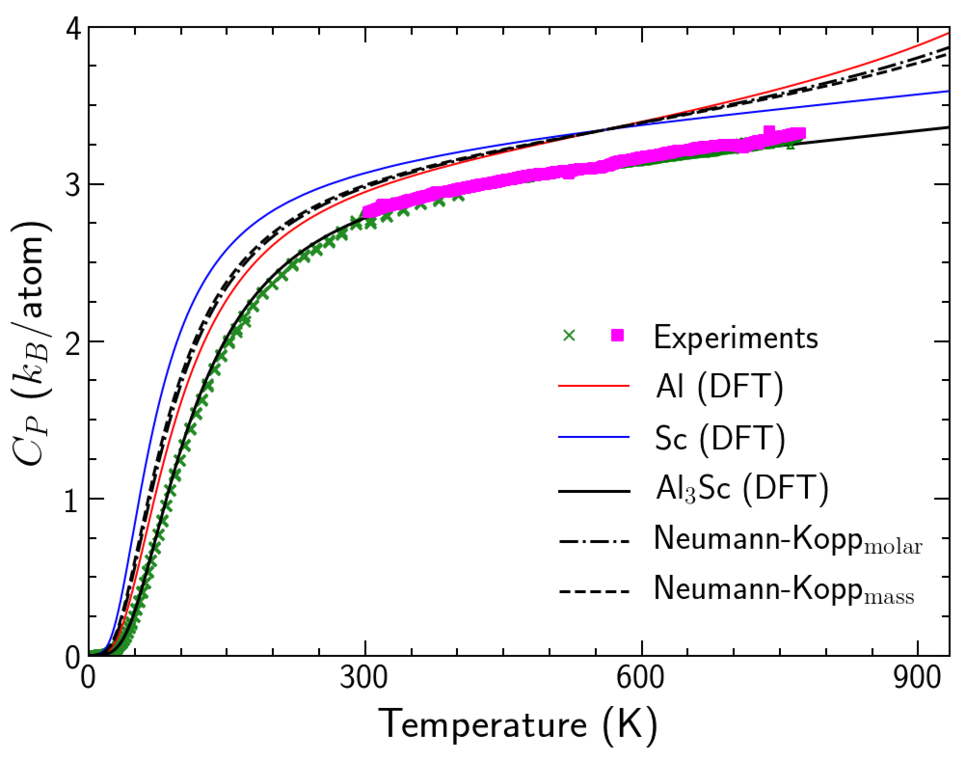
We are currently working on alternative approaches to the Neumann-Kopp rule in order to systematically extrapolate to multicomponent compounds. Our preliminary results below room temperatures for both Mg2Si and Al3Sc that correlate elastic and thermodynamic parameters look promising, but need to be verified with high-throughput methods. This analysis will be of practical interest for the third generation of CALPHAD approaches, especially for metastable compounds that are important for the databases but challenging to synthesize in laboratory.
References
[1] P.J. Spencer, A brief history of CALPHAD, CALPHAD 32 (2008)
[2] H.Zhang, Y.Wang, S.L. Shang, C.Ravi, C.Wolverton. L.Q.Chen, Z.K.Liu, CALPHAD 34 (2010)
[3a] B.Grabowski, T.Hickel, J.Neugebauer, Phys. Rev. B, 76 (2007)
[3b] B.Grabowski, L.Ismer, T.Hickel, J.Neugebauer, Phys. Rev. B. 79 (2010)
[4] Gerstein, Habenschuss, J. Chem, Phys. 47, 6 (1967)
[5] Feufel, Sommer, J. Alloys Compd 247, 31 (1997)
[6] Luedecke et. al., Z. Metallkd. 77, 278 (1986)
[7] Kevorkov et. al., J. Phase Eq. Diff. 25, 140 (2005)
[8] Feufel et. al., J. Alloys Compd. 247, 31 (1997)
[9] Yu et. al., J. Phys. Chem. Sol. 71, 758 (2010)
Acknowledgements
The research is performed in collaboration with the groups of I. Egry (DLR Köln), B. Hallstedt (RWTH Aachen), M. Rettenmayr (Uni Jena), and R. Schmid-Fetzer (TU Clausthal). Financial support of the Deutsche Forschungsgemeinschaft within the package project PAK461 is gratefully acknowledged.