Ir-TiO2 nanowires for electrochemical applications
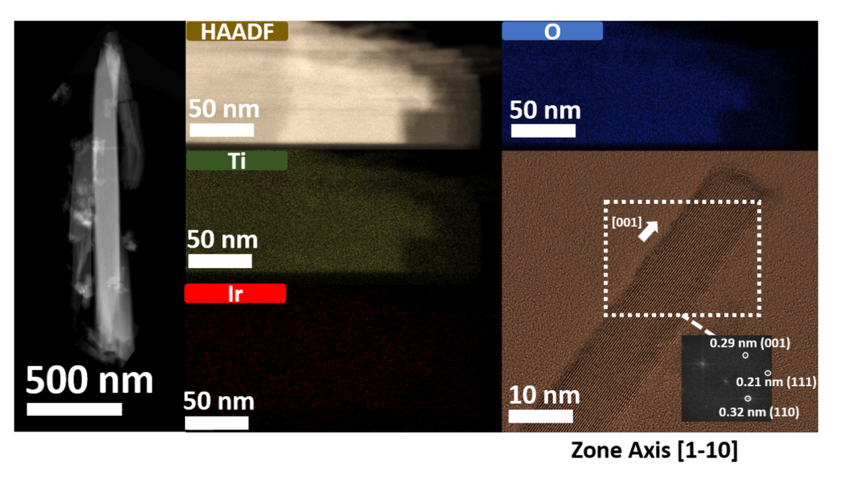
Single-crystalline TiO2 nanowires are utilized as stable support for iridium electrocatalysts. The local structure and interactions between the metal and the TiO2 support are analysed by electron microscopy These analyses are correlated to the electrochemical activity, effectively establishing synthesis-structure-property relationships at sub-nanometer scale.
Metal oxides possess high chemical stability, which make them attractive supports for metal electrocatalysts. However, the final properties of the supported catalysts depend on several parameters such as the local structure, chemistry and interactions between the metal and the support. Thus, efficient design of supported catalysts requires systematic studies to define the structure-property relationships at the sub-nanometer scale.
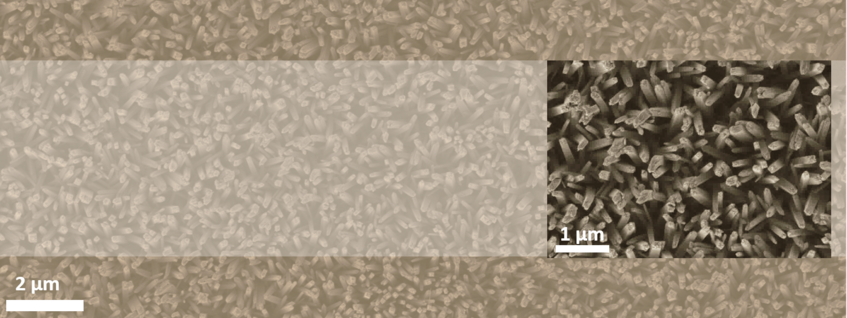
This project focuses on the hydrothermal synthesis and structural investigation of highly crystalline Ir-TiO2 nanowire arrays as supported electrocatalysts for the oxygen evolution reaction (OER). This synthesis method yields homogeneously dispersed Ir atoms with tunable Ir:Ti compositions. Emphasis is made on understanding the adsorption or bonding nature of the Ir metal atoms within the TiO2 support Finally, the Ir-TiO2 nanowires can be further modified by reductive annealing and etching processes with the aim to improve their conductivity and enhance their surface area for an optimized OER activity.
The structure and chemistry of the Ir-TiO2 nanostructures are analysed by advanced (scanning) transmission electron microscopy ((S)TEM), energy dispersive X-ray (EDS) and electron energy loss spectroscopy (EELS) techniques. The structural features of the nanomaterials are correlated to their electrochemical activity for application in OER electrocatalysis. Similar studies have been performed on Pt nanoparticles on hollow TiO2 supports [1] and to detect impurities within hollow TiO2 nanostructures where in addition to STEM also atom probe tomography was done [2].