How interstitial ordering affects high-strength steels
Scientists from the Max-Planck-Institut für Eisenforschung and the Ruhr-Universität Bochum publish their recent findings in Nature Materials
The performance of materials is strongly influenced by their alloying elements: Adding elements beyond the basic composition of the alloy can strongly influence the properties and performance of it. In practice, it is not only important which elements are added, but also to which amounts and how they order in the host lattice. For the fundamental basic composition of any steel – iron and carbon - the concentration and ordering of carbon atoms and their interaction with the iron host lattice in martensitic steels was analysed by a team of scientists from the Max-Planck-Institut für Eisenforschung (MPIE) and the Ruhr-Universität Bochum (RUB). The scientists examined the mechanisms of collective interstitial ordering in Fe-C steels and determined how anharmonicity and segregation affect the ordering mechanism and consequently, the material’s performance. Their recent findings were published in Nature Materials.
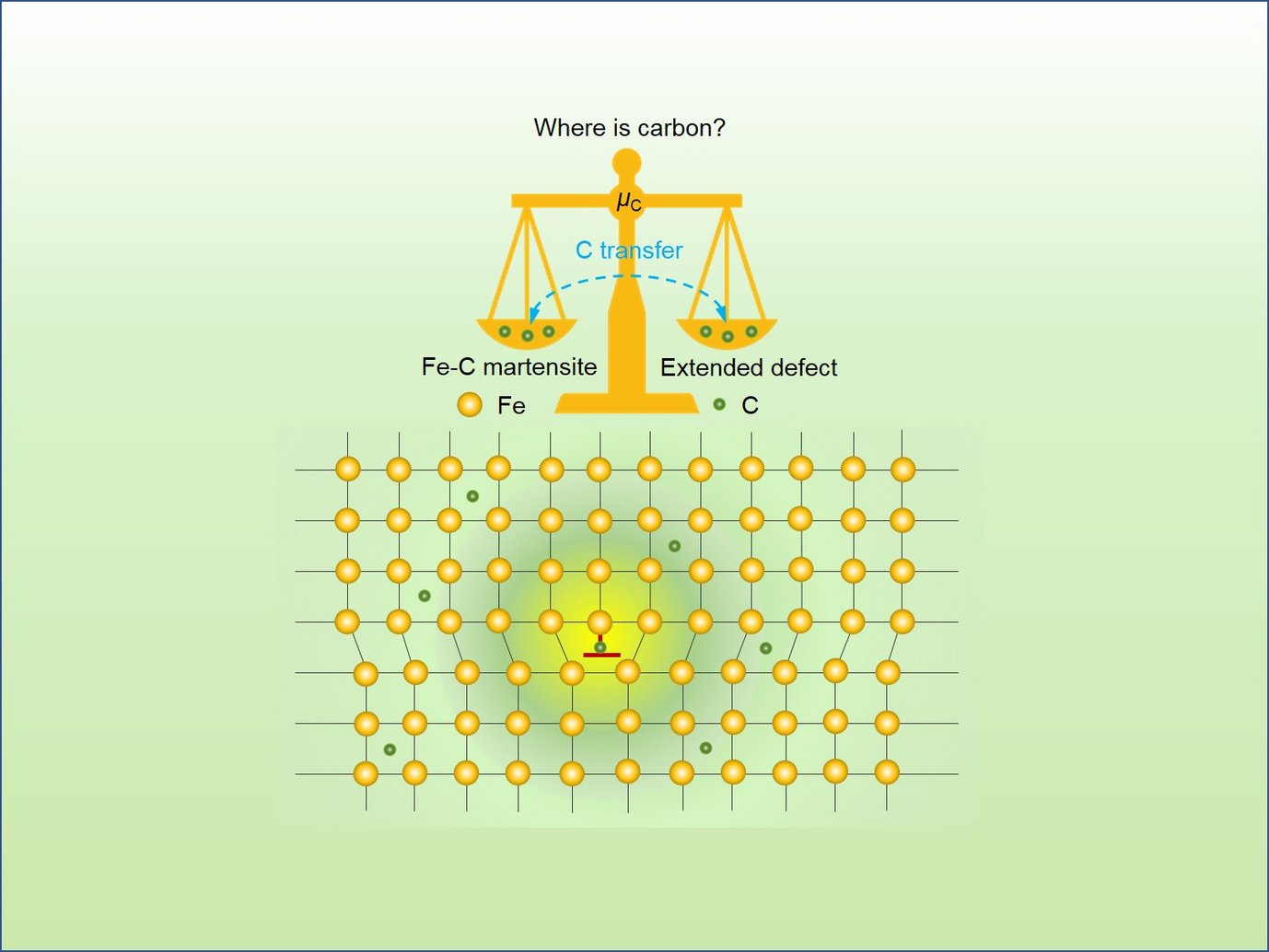
“When carbon atoms enter the iron host lattice of martensitic steels, they diffuse between the iron atoms and do not take over the iron atoms’ positions in the host lattice. Nevertheless, they create strain fields influencing the whole lattice. Understanding the mechanism of the resulting interstitial ordering is a key to designing ultra-high performance steels as they gain their strength from the martensite formation, thus, from the collective interstitial ordering”, explains Dr. Tilmann Hickel. Hickel is head of the group “Computational Phase Studies” at the MPIE and was the main supervisor of Dr. Xie Zhang, the first author of the publication. Each interstitial atom, due to its size and chemical interaction with atoms of the host lattice, creates a local strain field that displaces its neighbouring host atoms away from their original lattice positions. “Imagine inserting a wooden stick into sand at the beach and watching how the stick displaces the grains of sand surrounding it. The same happens when we add carbon to the iron host lattice. The carbon interstitials, find their way through the host lattice, order in energetically favourable places and distort and harden the previous structure.”, explains Hickel. A high concentration of interstitials leads to ordering/disordering phenomena and lattice distortions, thus influencing the steels’ bulk performance.
The research team identified two components that influence the interstitial ordering. The first one results from the anharmonicity caused by the strain fields in the Fe lattice. “Due to this anharmonicity, the critical C concentration for an order-disorder transformation is decreased. To understand the displacement of the Fe atoms at different distances, we must consider the anharmonic contribution in the first neighbour position of a C interstitial.”, explains Dr. Jutta Rogal from the Interdisciplinary Centre for Advanced Materials Simulation of the Ruhr-Universität Bochum.
The second component that influences the interstitial ordering is the segregation of C to extended defects. This segregation takes place at low C concentrations and is suppressed at high C concentrations due to a lowering of the C chemical potential in ordered martensite. The chemical potential of C in Fe-C martensite gradually increases with increasing C concentration until 0.8 at.% are reached. Then it rapidly decreases due to the order-disorder transition.
Both components, the level of anharmonicity and the segregation behaviour, are decisive for the order-disorder transition. “An unexpected outcome of the study was that it is not sufficient to analyse only the arrangement of the carbon atoms in bulk. Rather, a strong competition between the carbon concentration in the bulk and its segregation to extended defects occurs. Only with this insight it was possible to gain a comprehensive understanding of the order-disorder transition. This competition decreases with an increasing concentration of carbon interstitials, as extended defects can incorporate interstitials only to a limited amount. The exact concentration depends on the density of the defects. In our calculations and confirmed by experiments, disordered martensite is triggered by a carbon concentration in the range between 0.8 at.% and 2.6 at.%. Above 2.6 at.% ordered martensite is formed, which provides a superior strength to steels. Below 0.8 at.%, carbon atoms segregate to dislocations in grain boundaries”, explains Prof. Jörg Neugebauer, director of the department Computational Materials Design at the MPIE. The theoretical calculations were confirmed by transmission electron microscopy and atom probe tomography measurements performed at the Ruhr-Universität Bochum.
In general, the exact critical C concentration depends on the microstructure of the material and the binding energy between C and a specific extended defect. The shown critical concentration range of 0.8 at.% and 2.6 at.% is not universal, but depends on the sample and its extended defects. However, the critical concentrations can be precisely calculated if a) the exact binding energy between C and the extended defect, and b) the maximum C concentration that can be included by the extended defect, are known. The MPIE and RUB team showed the decisive role anharmonicity and segregation play regarding the mechanism of interstitial ordering, using the Fe-C alloys as a model for other relevant systems. Including anharmonic effects into order-disorder phase transitions provides a new level of predictive materials modelling, paving the way to designing ultra-high-performance steels.
The research was supported by the German Research Foundation within the DFG-ANR project C-TRAM.
Author: Yasmin Ahmed Salem
