Designing stable electrocatalysts for a clean energy future
A team of scientists from the Max-Planck-Institut für Eisenforschung (MPIE), the Massachusetts Institute of Technology (MIT) and the Forschungszentrum Jülich (FZ) discovered core-shell nanoparticles based on platinum and an inexpensive carbide core as active, stable and cost-efficient electrocatalysts for the oxygen reduction reaction - the cathode reaction in a fuel cell. The recent findings were published in Nature Materials.
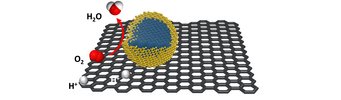
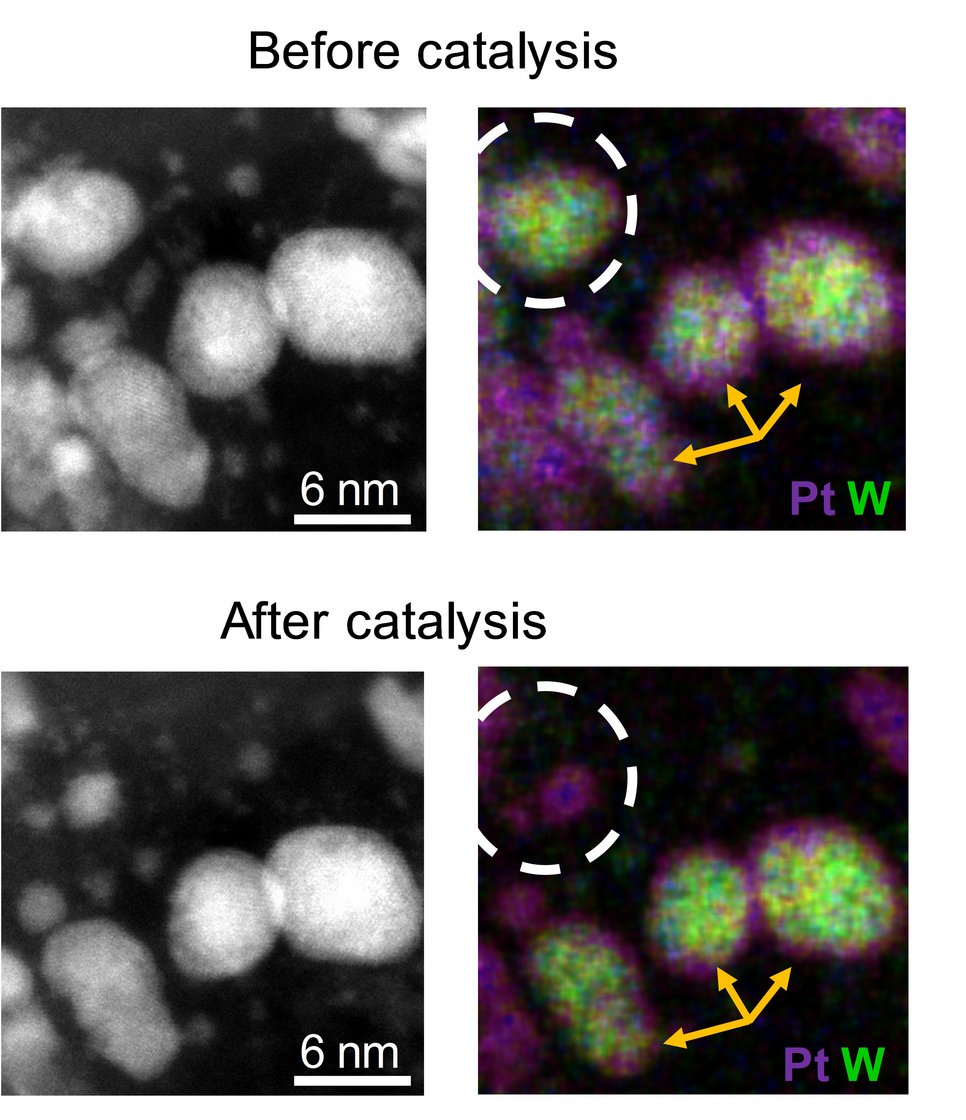
Polymer electrolyte fuel cells display one of the key elements for the transformation of chemical into electrical energy. However, the currently employed electrocatalysts still contain large amounts of noble metals due to the high durability requirements. This makes them cost- and resource intensive. A core-shell system as catalyst enables the reduction of precious metals while potentially increasing its activity and stability. It ideally combines an inexpensive and easily manufactured core with a shell made of a precious metal that is only a few monolayers thick. “We have found a way, to reduce the noble metal content up to 60-70% while simultaneously stabilizing the non-noble core. While the shells are made out of platinum, the cores consist of inexpensive metal carbides and nitrides. The employed catalysts are more stable during the oxygen reduction reaction compared to its bare noble-metal counterpart while offering similar activity at a lower material price.”, explains Daniel Göhl, doctoral student at the MPIE and first author, together with Aaron Garg from MIT, of the Nature publication.
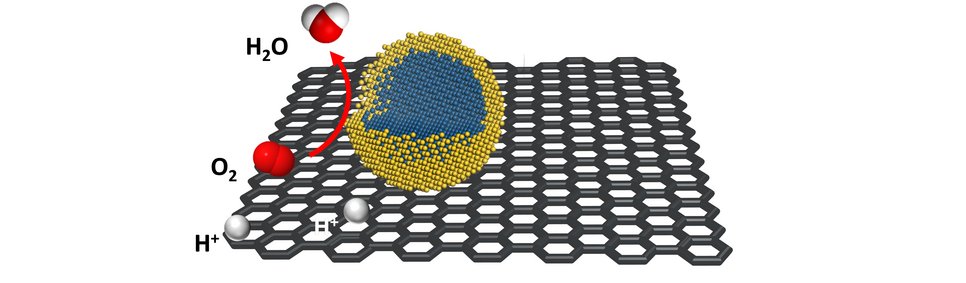
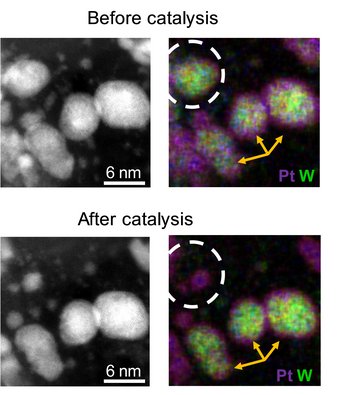
A special focus was laid on the degradation mechanism and the prerequisites to stabilize core-shell materials. The key for the successful evaluation was the coupling of electrochemical instruments with highly sensitive analysis tools. This includes in situ measurements with the scanning flow cell, a method which was developed at the MPIE, and ex situ identical location transmission electron microscopy at the FZ. The scientists found out that the catalyst retains the beneficial core shell structure over 10,000 degradation cycles depending on the homogeneity of the shell. Based on these results, the scientists aim to increase the amount of homogenous core-shell particles and to facilitate the synthesis. “The gained insights yield from a strong partnership between different institutions bringing together fundamental knowledge about the synthesis with an in-depth characterization with highest quality from various perspectives”, so Dr. Marc Ledendecker, one of the corresponding authors of the Nature publication and project coordinator of the project funded by the Federal Ministry of Economic Affairs and Energy.
The research at the MPIE was funded by the Federal Ministry of Economic Affairs and Energy and at the MIT by the Toyota Research Institute.
Author: Yasmin Ahmed Salem