Where is carbon?
How dislocations help to decompose cementite and supersaturate ferrite
A steel with great technological importance that critically depends on C-dislocation interactions are severely deformed pearlitic wires with up to 7 GPa strength. Experimental observations have revealed an exponentially increasing density of dislocations and a decomposition of cementite -the central iron car-bide in steels- during the severe plastic deformation. The original stoichiometric C-concentration of 25 at. % in cementite substantially decreases (to <10 at.%) and the C atoms redistribute within the ferrite lamellae. This redistribution results in a C supersaturation of ferrite, which is in extreme discrepancy with empirical predictions.
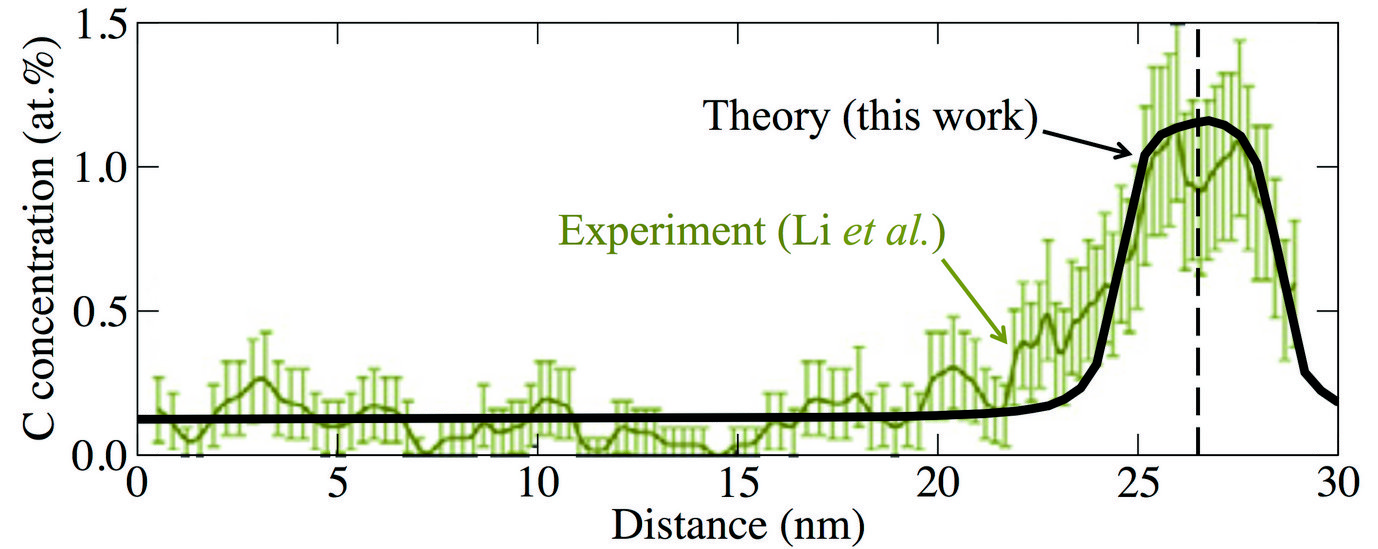
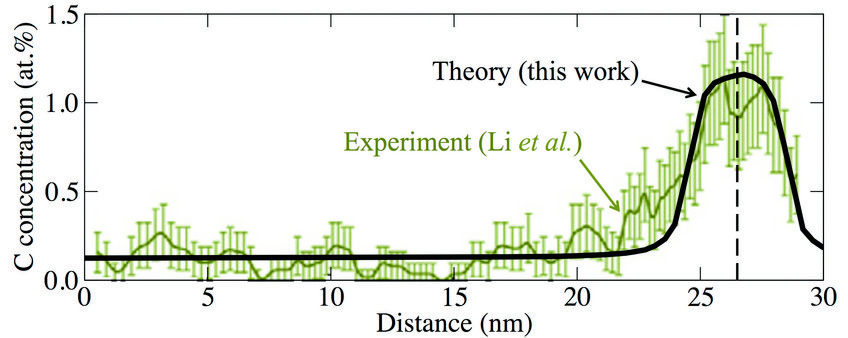
Fig. 2: Local carbon concentration around a defect (marked by the vertical dashed line) in a supersaturated ferrite phase after wire drawing (true strain ε = 2). Experimental data are from atom probe tomography measurements (Acta Mat 59 (2011) 3965).
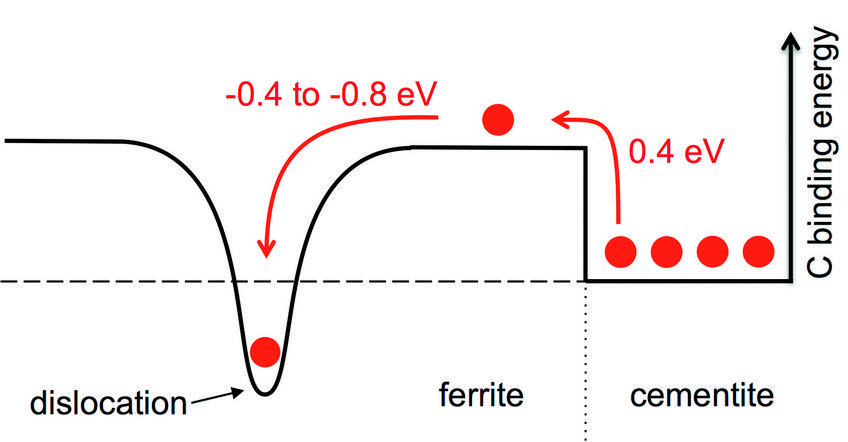
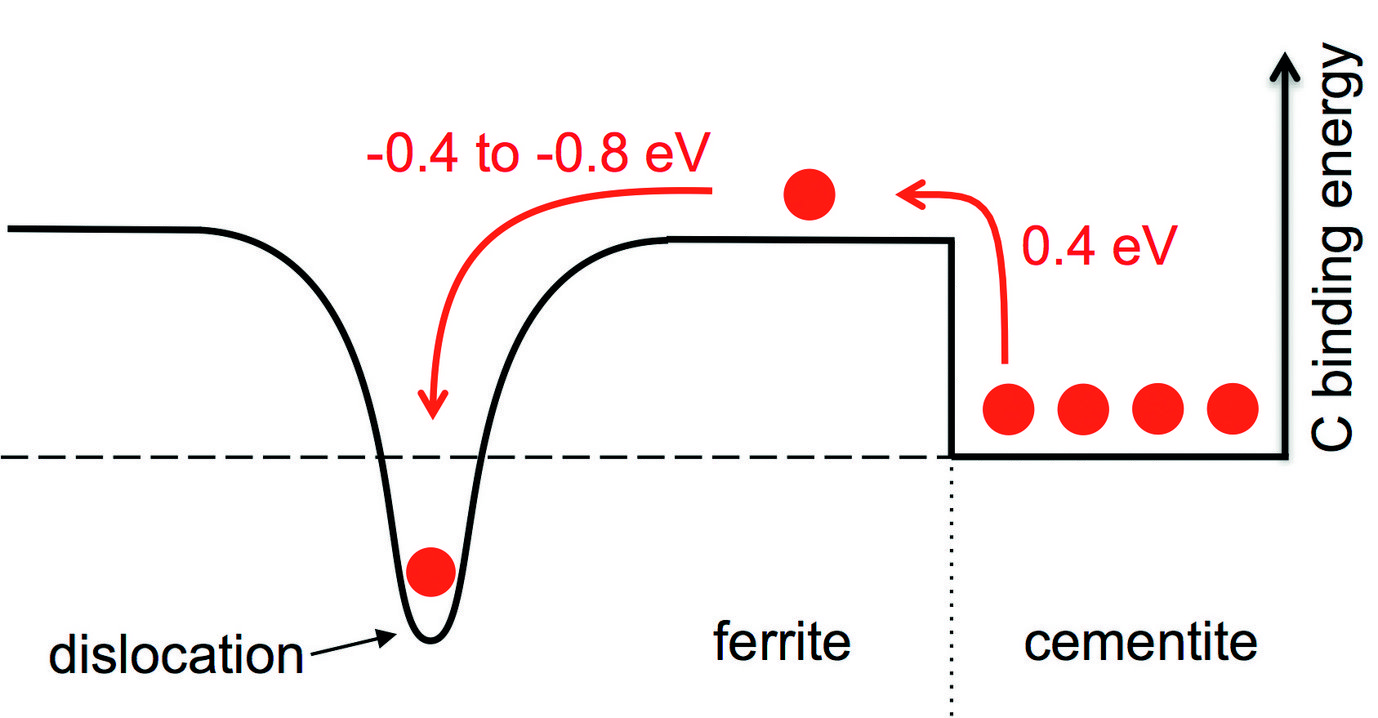
Resolving the discrepancy with experiment and empirical approaches alone is impossible due to the complex microstructure. We have therefore developed and applied a multiscale approach rooted in accurate atomistic simulations to tackle the problem. The main result of our calculations is schematically displayed in Fig. 1. Pearlite consists of ferrite (to the left of figure) and cementite (right) with C atoms (red balls) mainly placed in the cementite phase in thermodynamic equilibrium. To bring a C atom into the ferrite phase costs an energy of 0.4 eV which is too large to be overcome at realistic temperatures. However, the situation changes close to the dislocation (indicated by the dip in the solid black curve in Fig. 1), because of the strong C-dislocation binding energy in the range of -0.4 to -0.8 eV. The C-dislocation binding energy can compensate the original cost of bringing a C atom into ferrite, and the dislocation thus acts as an attractive sink for C atoms helping to decompose cementite and supersaturate ferrite.
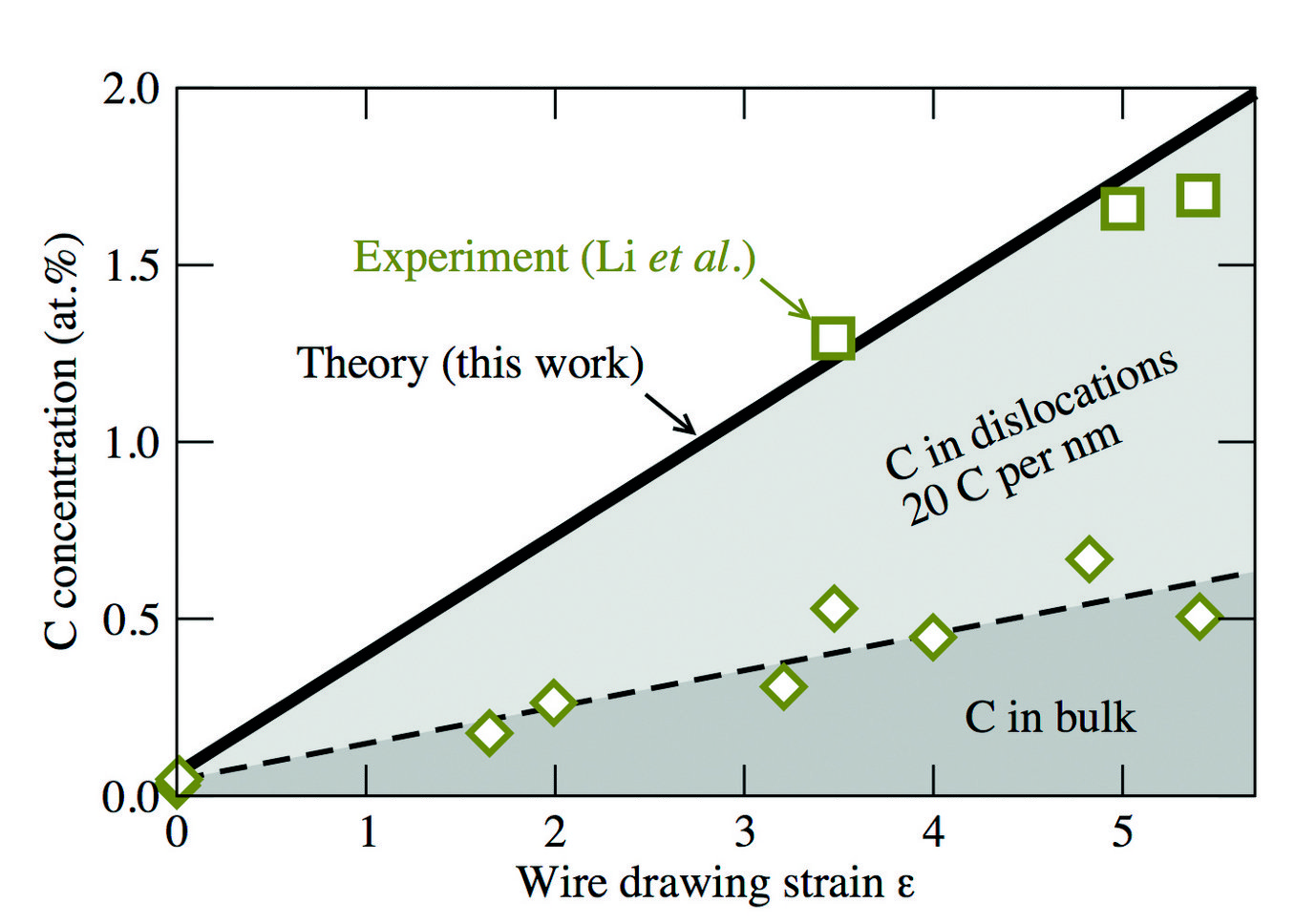
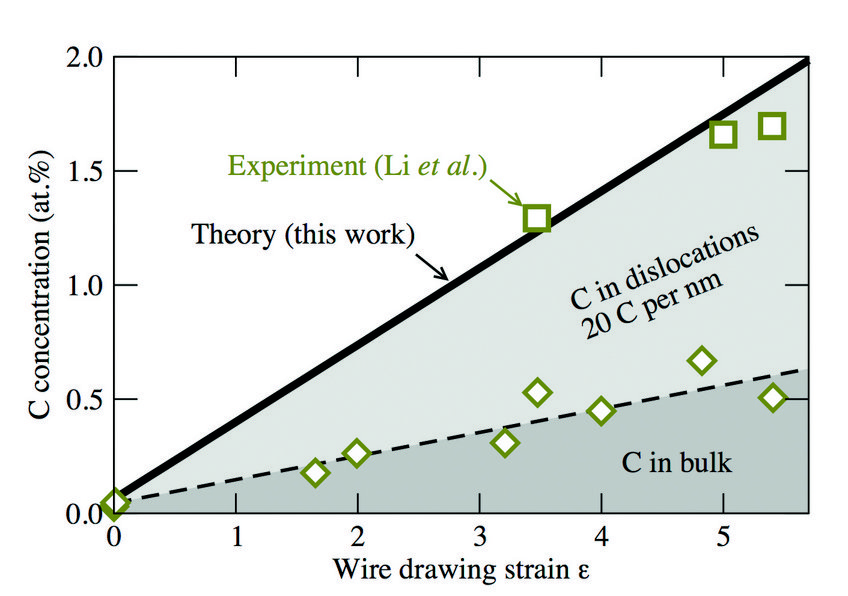
Fig. 3: Total carbon concentration (green squares and solid black line) in ferrite as a function of the true strain due to wire drawing. The green diamonds and the black dashed curve show the experimentally observed and calculated C concentrations, from the bulk lattice, i.e., excluding the contribution of dislocations.
Knowing the C-dislocation binding energies, we were able to calculate the C concentration profile around the dislocation by using an appropriate statistical (Fermi-Dirac) distribution. The result is shown in Fig. 2 (blackline) in comparison to experimental results (green data set) indicating a good agreement. By integrating over the computed C concentration profile in Fig. 2, we have derived the number of C excess atoms per dislocation. The result depends on the total C concentration, but a value of 20 C/nm of dislocation line length reflects a reasonable average. Using additionally the experimentally observed dislocation density, we estimated the increase in C content in the ferrite phase due to dislocations. The result is shown in Fig. 3 by the light gray shaded area. We observe a good agreement of our estimate (black solid line) with the experimentally determined concentrations (green squares), indicating that dislocations play an important role in stabilizing the C supersaturation of the ferrite phase during wire drawing.
Authors: Gh. Ali Nematollahi & Blazej Grabowski