Photocatalysts
The sunlight is capable of answering the global energy need. Various technologies are being developed to convert the sunlight into thermal energy, electricity (photovoltaic), or fuels.
Within the framework of SPP 1613, fuels produced regeneratively through light-driven water splitting, we and collaborators form a team to investigate the challenges to convert solar energy into the most promising fuel, H2, by the water splitting reaction, H2O → H2 + ½ O2.
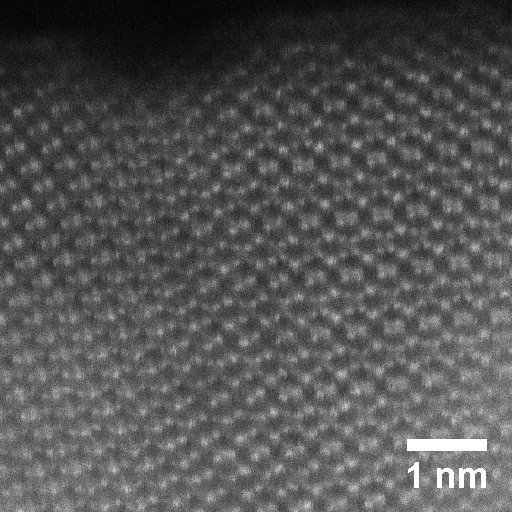
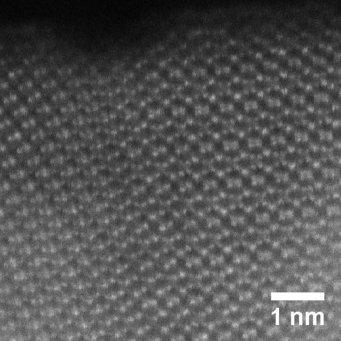
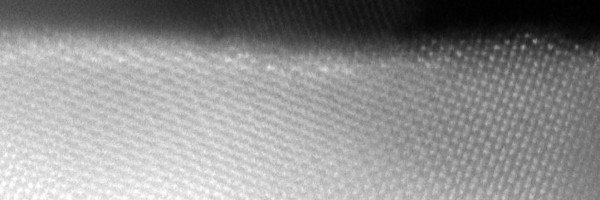
Semiconductors with a relatively big (> 1.5 eV) band gap are necessary to provide the thermodynamic driving force. We focus on oxide semiconductors that are stable in the electrochemical cells. Bulk oxides have low carrier mobility and high overpotentials for the water splitting reaction. To address such limits, our design strategies take advantage of nanostructures and alloying/doping chemistry.
Interfaces and surfaces respectively control the transport of the carriers and the environment for the water splitting reaction. We apply state-of-the-art electron microscopy to image the important interfaces and surfaces as well as to study their local chemical composition. This is applied to examine new nanostructures synthesised from the Bein group and the Fattakhova group. Moreover, the atomic resolved information is straightforwardly fed into the atomistic modelling from the Pentcheva group.