Chalcopyrite nanomaterials for renewable energy applications
In the light of growing energy needs while, at the same time, depleting fossil fuels, increasing environmental pollution and the threatening global warming, the search for alternative energy sources is a steadily growing field of research. Chalcopyrite materials proved being suitable for a wide range of different applications, for example solar cells, water splitting or photo catalysis. In our study, we synthesize copper indium disulfide, CuInS2, via a solvothermal procedure. By this method, nanostructured crystalline films can be grown at temperatures below 200 °C. The investigation of the as-grown films is mainly conducted via electron microscopic techniques and related spectroscopies.
In consequence of the growing energy needs of our society and the increasing environmental pollution due to fossil fuels alternative energy concepts are needed. Various approaches for the production of green energy include, but are not limited to, solar cells, water splitting, fuel cells, photo catalysis, etc. Among various suitable materials, copper indium disulfide, CuInS2, is a promising material as it offers a direct band gap of around 1.5 eV, combined with a high absorption coefficient of 105 cm−1, which allow the utilization of a broad range of the incoming sunlight. Another advantage of CuInS2 is that it is a hole or electron conductor depending on the chemical composition of the material, which opens up even more possible fields of application.
In our study, we chose a solvothermal synthesis strategy, as it allows to grow solid films already at temperatures below 200 °C due to the increased pressure inside a closed reaction vessel, so-called autoclaves (produced in-house, compare the teaser image). Additionally, the method requires only simple precursor salts or molecules as well as commonly used solvents, which makes the method easily accessible. Furthermore, by using the solvothermal synthesis it is possible to grow CuInS2 as a thin film directly on a transparent conductive oxide (fluorine-doped tin oxide, FTO) which eliminates an additional step of depositing the material after synthesis.
By tuning the reaction conditions (temperature, time) or the reagents (precursor salts/molecules, solvents, their concentration and/or ratio) the morphology, thickness, crystallinity of the as-synthesized material, and therefore its properties, can be triggered.
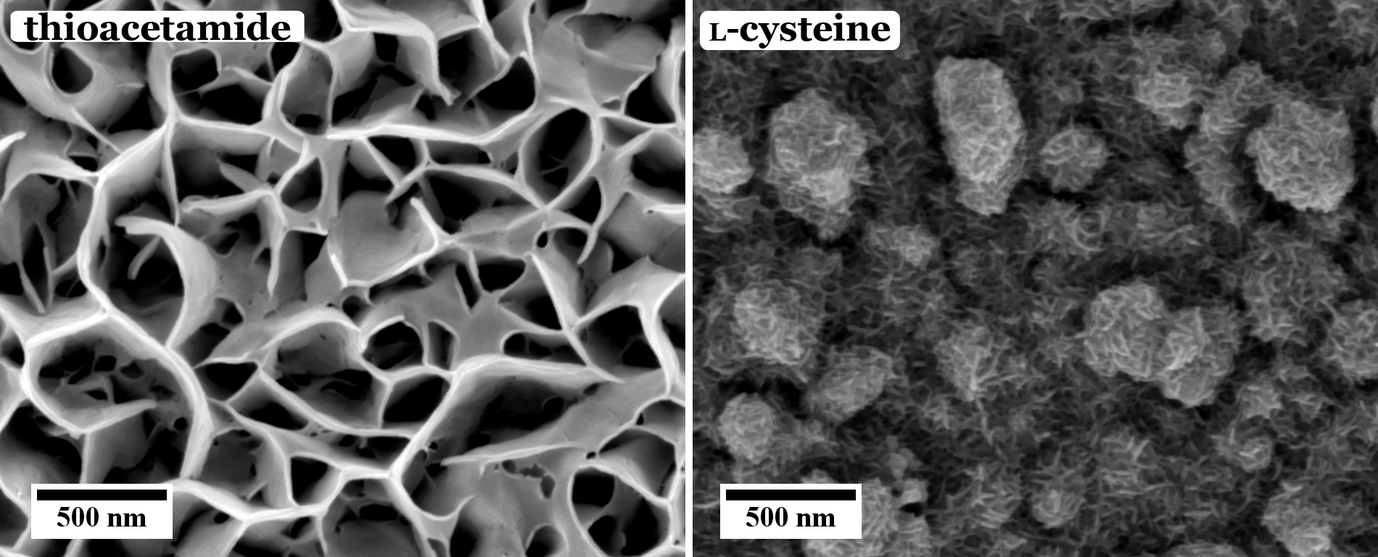
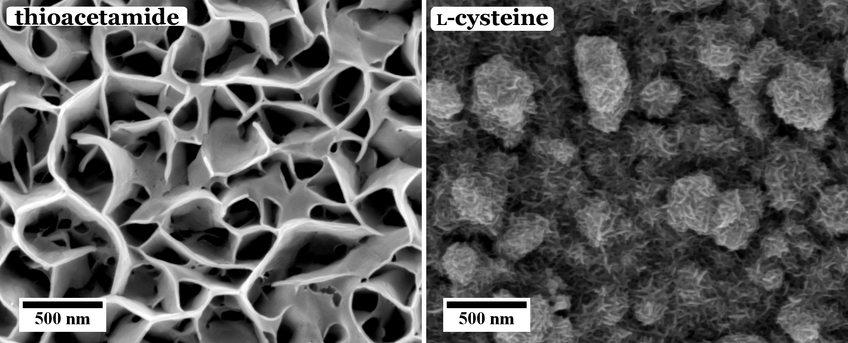
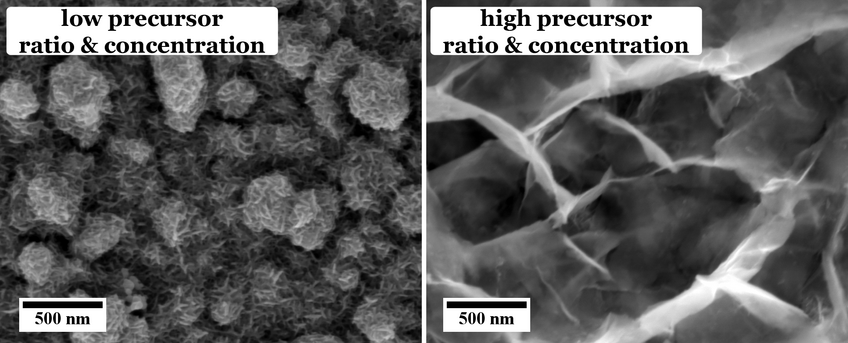
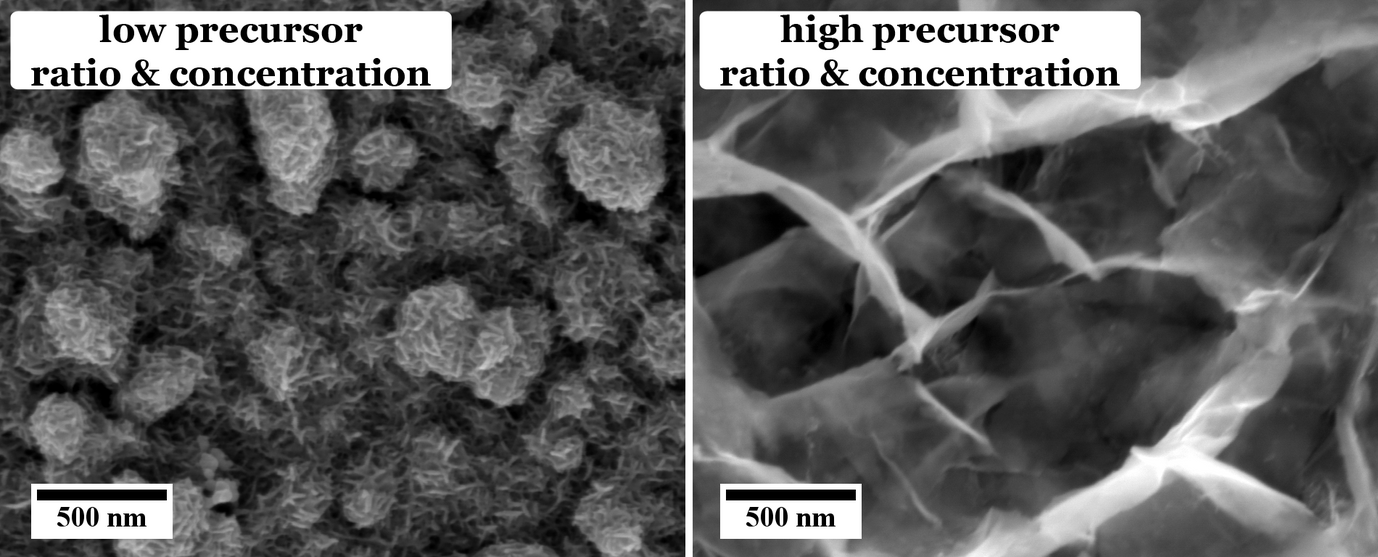
These different effects are investigated in our group. For example, the influence of different sulfur sources, i.e. organic thiol containing molecules, namely thioacetamide and l-cysteine, on the morphology and chemical composition of the CuInS2 films has been investigated. As can be seen from Figure 1, the use of thioacetamide as sulfur source leads to an open, flower-like surface morphology, while l-cysteine leads to a more compact CuInS2 film. Furthermore, we observed that for higher concentrations and ratios of the precursors the morphology as well as the chemical composition of the CuInS2 films grown with l-cysteine is changed (Figure 2).
Characterization of the as-grown CuInS2 samples is mainly done by electron microscopic techniques, namely scanning electron microscopy and (scanning) transmission electron microscopy as well as their related spectroscopic methods like energy-dispersive X-ray spectroscopy or electron energy-loss spectroscopy. The influence of the chosen sample preparation techniques (e.g. conventional polishing and ion thinning vs. focused ion beam milling) is also investigated. While grazing-incidence X-ray diffraction is applied to determine the overall crystallographic composition of the CuInS2 films, electron diffraction can provide more local information about the crystallinity. The absorption properties and band gap values of the grown CuInS2 films are analyzed by UV/Vis spectroscopy. To complete the characterization of the as-grown material also dye degradation studies under simulated solar illumination are conducted to gain first insights into the photocatalytic properties of the material.