Investigation of photo-electrochemical water splitting-active nanostructures
While solar cells are becoming more and more widespread, storing the produced energy is difficult. Of the many possible solutions, the splitting of water into H2 and O2 by a photoelectrochemical cell offers an elegant approach. The hydrogen can then easily be stored, transported and burned in a fuel cell. As most features in photoelectrodes are on the nano- or microscale, electron microscopy is very powerful. We study material systems such as Fe2O3 or TiO2 and attempt to identify performance bottlenecks.
The last few years have seen a surge of research in fields related to renewable energy. Solar energy in particular is a promising contender as it is a nearly endless resource which could easily power all of the earth’s energy needs. However, storage of solar power to compensate for night, cloudy conditions and so forth is a challenge. Of the many possible storage options, the conversion of solar energy into chemical energy via the splitting of water into hydrogen and oxygen offers an elegant and practical approach. The resulting hydrogen can easily be stored, transported and used in a fuel cell to regain electrochemical energy.
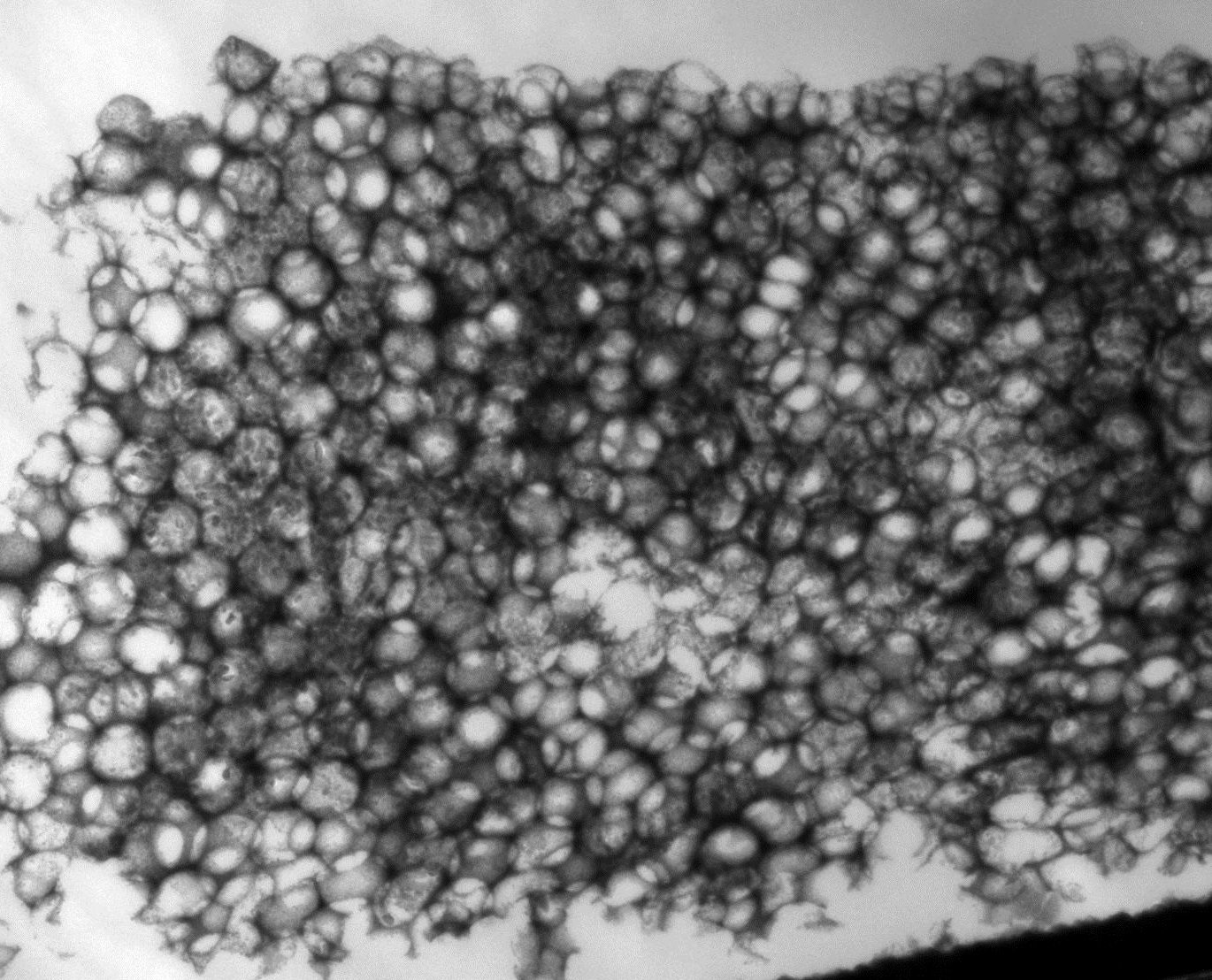
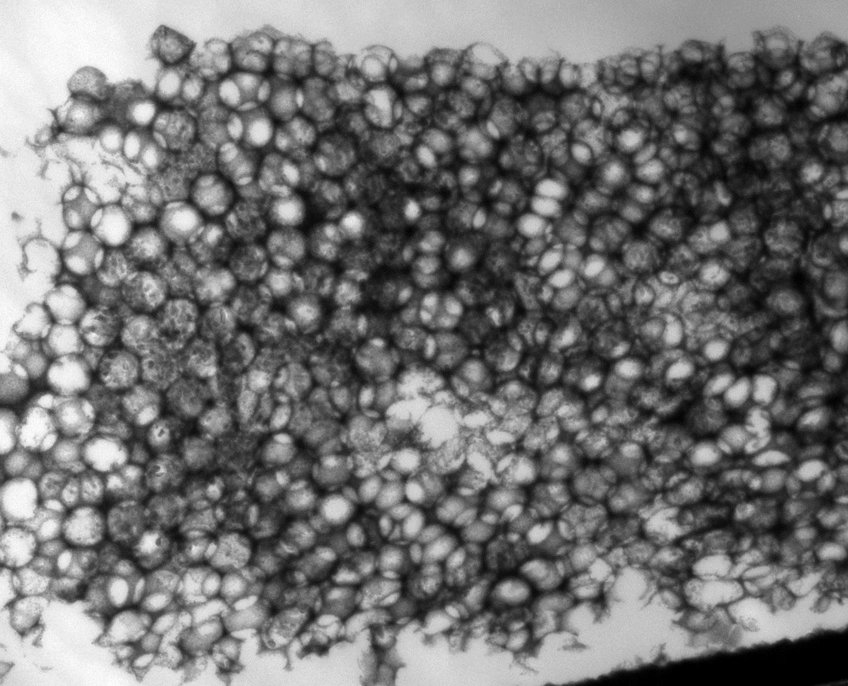
Unfortunately, this goal currently is far from reached as even the best photoelectrodes are not efficient enough to be economically viable. Apart from a search for new material systems, many strategies to improve already known, promising systems are being attempted. Among them are doping, surface modifications, different morphologies and so on. As these modifications are on the nano- to microscale, transmission electron microscopy (TEM) and related techniques such as electron energy loss spectroscopy (EELS) and energy-dispersive X-ray spectroscopy (EDX) are very powerful analysis methods, and different material systems such as hematite and Au@TiO2 are being studied to correlate the atomic arrangement and chemical composition with the properties of the photoanode.