Unveiling challenges in green hydrogen production
International researcher team reveals adverse effects of hydrogen on catalysts. Their results are featured as the cover paper in the August issue of the journal ACS Energy Letters
Longevity and performance of electrocatalysts for the production of green hydrogen depends on the occurence of boron in the microstructure.
The quest for hydrogen as a clean and sustainable energy source has gained momentum. To produce green hydrogen, water must be split into oxygen and hydrogen. This water splitting process is facilitated by electrocatalysts that enhance the chemical reaction rate. Ideally, a catalyst is neither changed nor degraded by the reaction, and for electrolysers this becomes critical as the electrocatalysts account for 50% of its total cost. As a result, their efficiency and lifetime are critical to the future availability of green hydrogen and thus to a carbon-free economy. A team of researchers led by the Max-Planck-Institut für Eisenforschung (MPIE) has discovered why these catalysts actually deteriorate and suffer from a shorter life expectancy. Their work shows that the produced hydrogen itself is the bottleneck. The scientists have now published their findings in the journal ACS Energy Letters.
Effects of hydrogen on catalytic performance
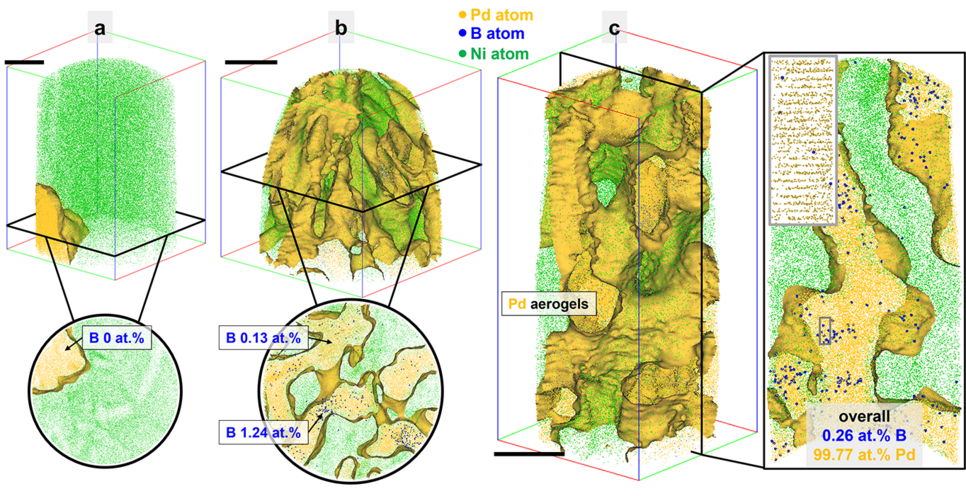
Previous research primarily focused on optimizing catalyst performance, without atomic-level analysis. However, the Max Planck team took a different approach. “Our findings revealed that impurities do get introduced during synthesis. Surprisingly, we discovered that boron impurities could enhance the catalyst's performance by expanding its lattice structure. However, we observed that the catalytic activity decreases after a certain amount of hydrogen is produced and wanted to understand why this happens to find ways to maintain the performance”, explains Prof. Baptiste Gault, corresponding author of the publication and head of the group “Atom Probe Tomography” at MPIE. Atom probe tomography and simulations based on density functional theory revealed that as hydrogen accumulates on the catalyst's surface, boron is gradually removed from the lattice structure. This interaction deteriorates the catalyst's performance, by decreasing the concentration of boron dopants.
Next steps: Protecting catalytic dopants from hydrogen
“Our findings show that it is not enough to increase the catalytic activity with Boron as a dopant. We must find solutions to shield Boron inside the catalyst’s lattice structure from the hydrogen produced on the surface of the catalyst”, says Prof. Se-Ho Kim, second corresponding author of the publication, former postdoctoral researcher at MPIE and now assistant professor at Korea University.
This research was made possible through funding from the European Research Council as part of the Shine project, led by Gault. It represents a major step forward in our understanding of green hydrogen production, paving the way for a more sustainable and cost-effective future in renewable energy.
Author: Yasmin Ahmed Salem
