Degradation studies on polymer-based fuel cells
To prevent global temperature from rising over 1.5 °C, it is critical to decarbonize our energy system by replacing fossil fuels with more green alternatives. In the transition to a world powered by renewable energy sources, fuel cell technology can have a major role. This project focuses on studying the degradation suffered by polymer electrolyte membrane fuel cells during operation, one of the biggest issues for their widespread commercialization.
Fuel cells (FCs) are devices that convert the chemical energy stored in a fuel (typically hydrogen or methanol) into electrical energy via electrochemical reactions on their electrodes. In this regard, they are very similar to batteries. However, unlike batteries, FCs are not closed systems. Therefore, they require a continuous supply of fuel and oxidizing agent. On the way towards maximum FC efficiency, it is key to understand the degradation processes that take place during operation, since they result in a drop in FC performance and ultimately limit FC lifetime. These degradation processes range from the diffusion/agglomeration of the catalyst species to the decomposition of the support materials. To comprehend the degradation processes and refer them to their cause, FCs before and after operation are investigated, either in real stack tests or using accelerated conditions. Different catalyst and catalyst support materials are tested to determine the relationship between FC efficiency, microstructural changes and operational degradation. This is done in close collaboration with our industry partner Freudenberg Fuel Cell e-Power Systems GmbH as part of the BMWi funded project “PAULL”. The ultimate goal is to identify material systems with minimal degradation to achieve a long live time of the device.
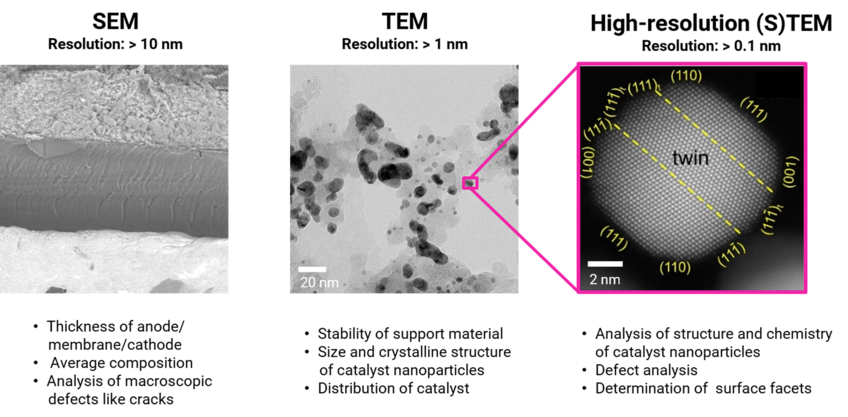
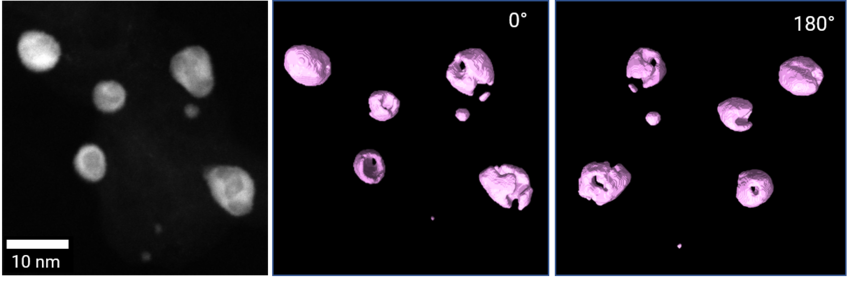
The FCs are characterized at multiple length scales (Figure 1). Scanning electron microscopy (SEM) techniques are used for studying the average composition of the electrodes and assessing their thickness and morphology. Conventional transmission electron microscopy (TEM) allows for the determination of the size and crystalline structure of the catalyst nanoparticles (NPs), together with their distribution on the support material. At higher magnifications, high resolution (scanning) TEM (HR-(S)TEM) techniques are used to locally study the chemistry and crystalline structure of the catalyst NPs, analyze their defects and determine the surface facets. Other advanced electron microscopy techniques applied in our studies include energy dispersive X-ray spectroscopy (EDS), electron energy loss spectroscopy (EELS) for probing the degree of sp2/sp3 hybridization of the support material and electron tomography, for the 3D visualization and characterization of the catalyst.
Furthermore, we are currently implementing correlative STEM-atom probe tomography (APT) experiments. By combining the high spatial resolution of STEM with the extremely sensitive, 3D chemical information obtained with APT, we can study the presence of impurities in the catalyst NPs, which are known to be detrimental to their catalytic activity.